Embed presentation
Downloaded 16 times
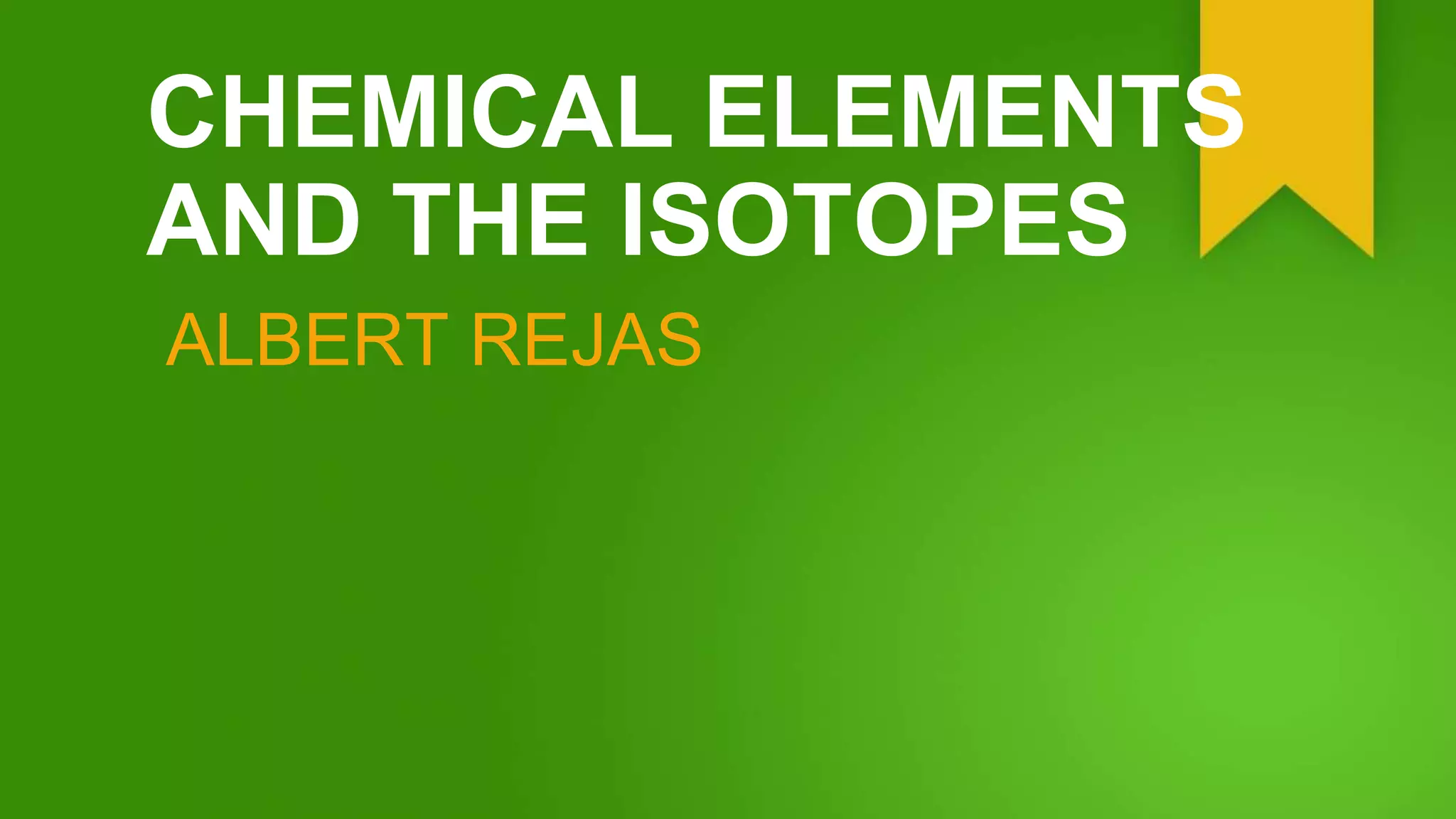
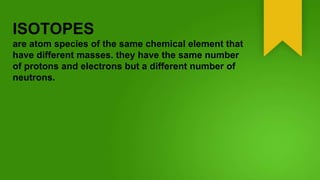

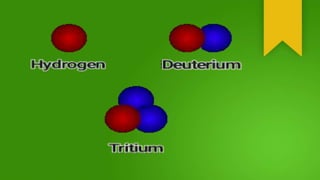


Isotopes are atom species of the same chemical element that have different masses due to varying numbers of neutrons, while having the same number of protons and electrons. The element hydrogen has three main isotopes: regular hydrogen with one proton; deuterium with one proton and one neutron; and tritium with one proton and two neutrons. Isotopes of the same element typically behave the same chemically, though some are radioactive and decay by emitting radiation while converting to a different isotope or element. Different isotopes are believed to have been created during the Big Bang or in supernova explosions and cosmic ray collisions.





