A research team from a university found a fossil at a location in Singapore and used carbon dating to determine its age. Carbon dating works by measuring the ratio of carbon-12 and carbon-14 in organic material, since carbon-14 decays at a known rate. The team used a logarithmic formula involving the natural logarithm of the carbon-14 percentage in the sample compared to a living organism, multiplied by the carbon-14 half-life of 5,700 years, to calculate the fossil's age. They provided an example calculation where a sample with 10% carbon-14 was determined to be 18,940 years old.




![LOGARITHMS
•A formula to calculate how old a sample is
by carbon-14 dating is:
•t = [ ln (Nf/No) / (-0.693) ] x t1/2
•t = [ ln (Nf/No) / (-0.693) ] x t1/2
•where ln is the natural logarithm, Nf/No is the
percent of carbon-14 in the sample
compared to the amount in living tissue, and
t1/2 is the half-life of carbon-14 (5,700 years).](https://image.slidesharecdn.com/logarithmscarbondating-140530015004-phpapp02/85/Group-3-Logarithms-carbon-dating-5-320.jpg)
![AN EXAMPLE
• So, if you had a fossil that had 10 percent carbon-14
compared to a living sample, then that fossil would
be:
• t = [ ln (0.10) / (-0.693) ] x 5,700 years
• t = [ (-2.303) / (-0.693) ] x 5,700 years
• t = [ 3.323 ] x 5,700 years
• t = 18,940 years old](https://image.slidesharecdn.com/logarithmscarbondating-140530015004-phpapp02/85/Group-3-Logarithms-carbon-dating-6-320.jpg)


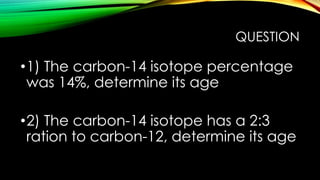
![WORKED SOLUTIONS
• 1)
• t = [ ln (0.14) / (-0.693) ] x 5,700 years
• t = [ (-1.966) / (-0.693) ] x 5,700 years
• t = [ 2.837 ] x 5,700 years
• t = 16,170years old](https://image.slidesharecdn.com/logarithmscarbondating-140530015004-phpapp02/85/Group-3-Logarithms-carbon-dating-10-320.jpg)
![WORKED SOLUTIONS
• 2)
• t = [ ln (0.40) / (-0.693) ] x 5,700 years
• t = [ (-0.916) / (-0.693) ] x 5,700 years
• t = [ 1.322 ] x 5,700 years
• t = 7536 years old](https://image.slidesharecdn.com/logarithmscarbondating-140530015004-phpapp02/85/Group-3-Logarithms-carbon-dating-11-320.jpg)
