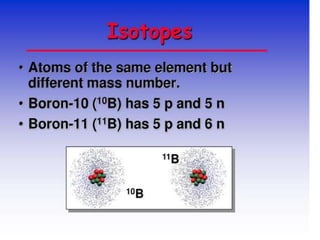
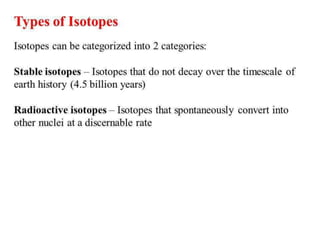
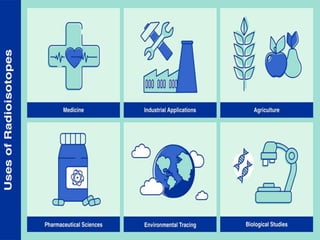
Isotopes are atoms of the same element that have different numbers of neutrons. There are two main types: stable isotopes that do not decay over time, and unstable or radioactive isotopes that decay by emitting radiation. Isotopic signatures can be used to trace the origins and migration of materials. Examples of isotope uses include carbon-14 dating of fossils, uranium-235 as nuclear fuel, and radioactive isotopes for medical purposes like cancer treatment and diagnosing thyroid problems.