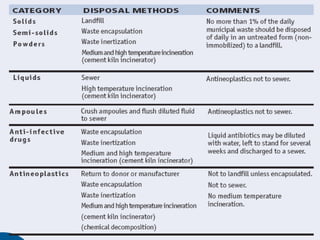
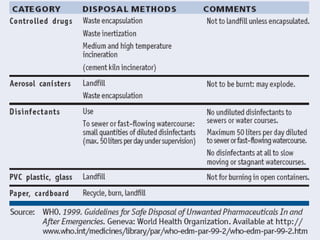
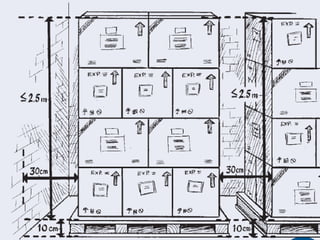
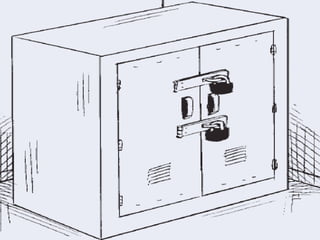
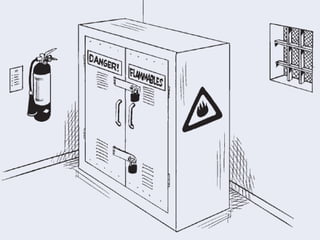
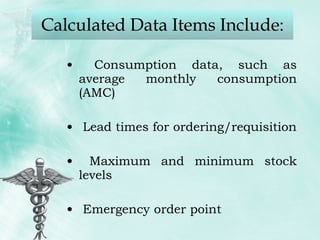
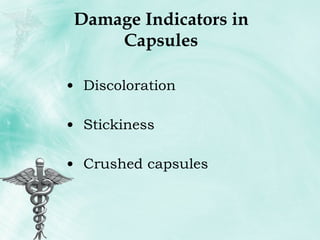
The document provides guidance on good storage practices for a medical store or storeroom. It discusses routine management tasks that should be done daily or weekly like cleaning, monitoring storage conditions and security. It also outlines procedures for receiving shipments, arranging products following FEFO policy, and specific storage requirements for controlled substances, flammables, and corrosives.
































































![The minimal information that should be collected on stock records for medicines and other health products includes: Product name/ description (including the form [e.g., capsule, tablet, liquid suspension, etc.] and strength) Stock on hand/ beginning stock balance](https://image.slidesharecdn.com/introductiontogoodstoragepracticesfull-100702102420-phpapp02/85/Introduction-to-good-storage-practices-full-70-320.jpg)























































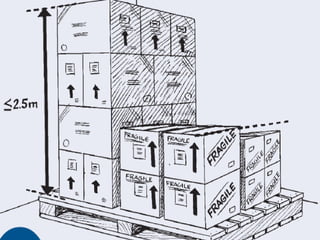



























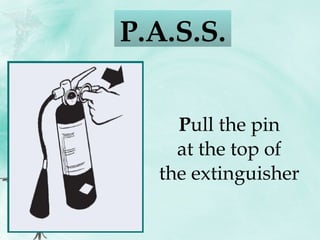

![S queeze the handle to discharge the extinguisher (stand approximately 2.5 m [8 ft] away) P.A.S.S.](https://image.slidesharecdn.com/introductiontogoodstoragepracticesfull-100702102420-phpapp02/85/Introduction-to-good-storage-practices-full-159-320.jpg)