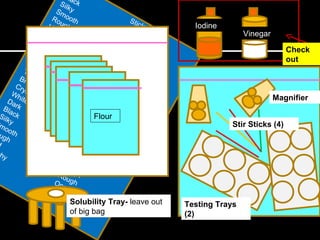
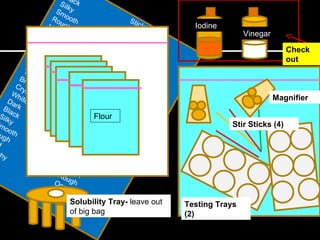
This document provides instructions for a chemistry lab involving testing unknown powder samples. Students are asked to:
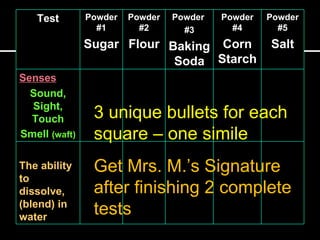
1) Perform a series of tests (sensory observations, solubility, iodine, vinegar, heat) on 5 known powder samples and record results.
2) Use the evidence from the tests to determine the identities of 2 unknown powders in a sample bag.
3) Explain their reasoning for the identifications and turn in their lab sheet. Proper lab clean-up and binder/note submission are also required.