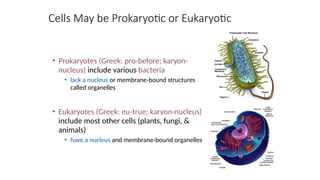
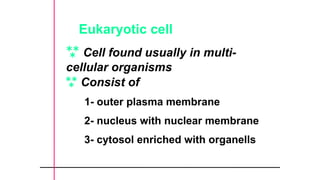
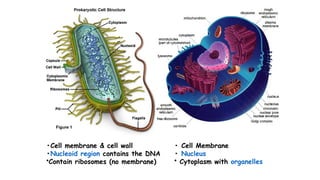
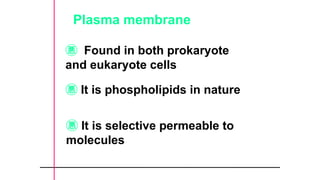
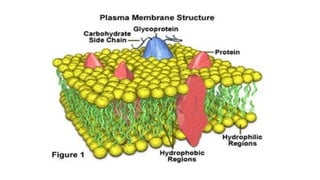
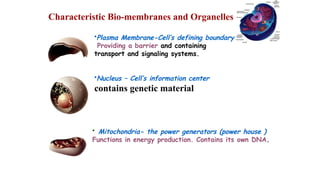
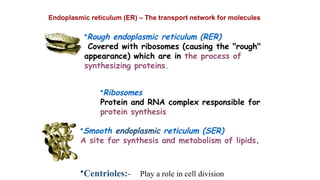
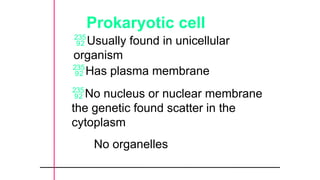
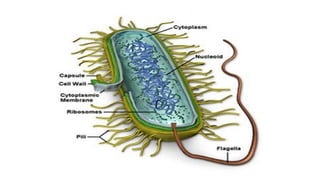
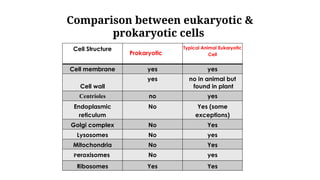
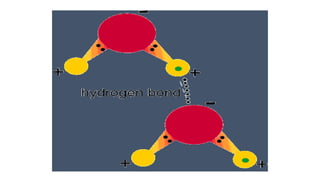
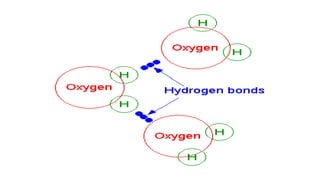
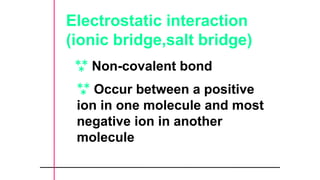
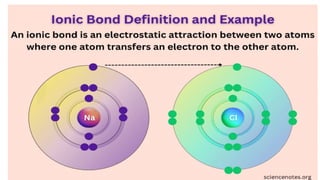
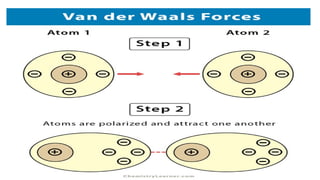
The document provides an introduction to biochemistry, defining it as the chemistry of life and detailing the importance of understanding biomolecules and their reactions in living systems. It describes the differences between prokaryotic and eukaryotic cells, highlighting their structures and key organelles, as well as the role of biomolecules such as proteins and lipids in cellular processes. Additionally, it discusses the significance of biochemistry in medicine and pharmacology, emphasizing the biochemical basis of diseases and drug action.