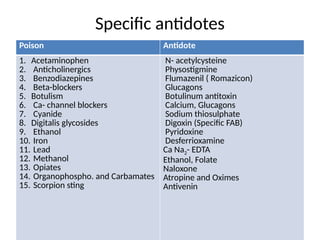
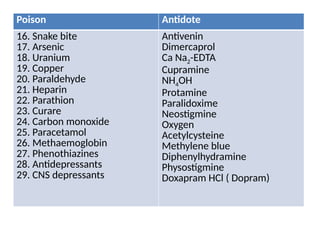
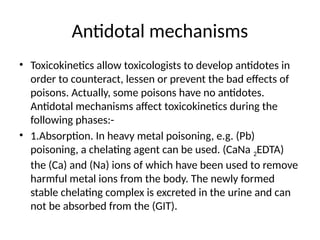
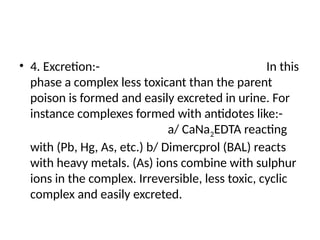Antidotes are agents that either remove poisons, prevent their absorption, or counteract their effects, classified into physical, chemical, and physiological types. Various antidotes work through mechanisms like adsorption, chemical neutralization, and chelation, each suited for specific poisons, and may involve compounds like activated charcoal and EDTA. The document also details specific antidotes for various poisons, their administration routes, and mechanisms of action.