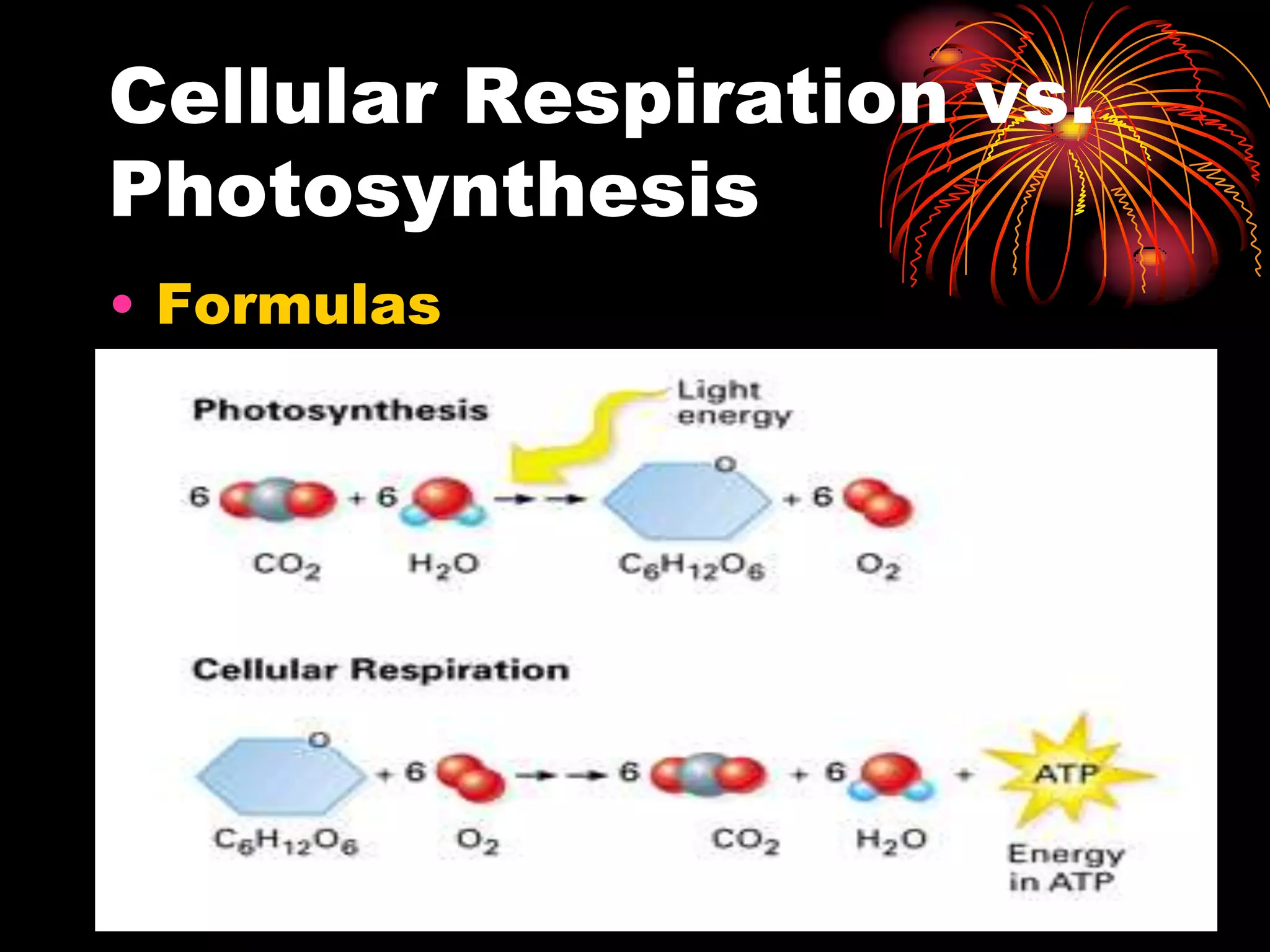
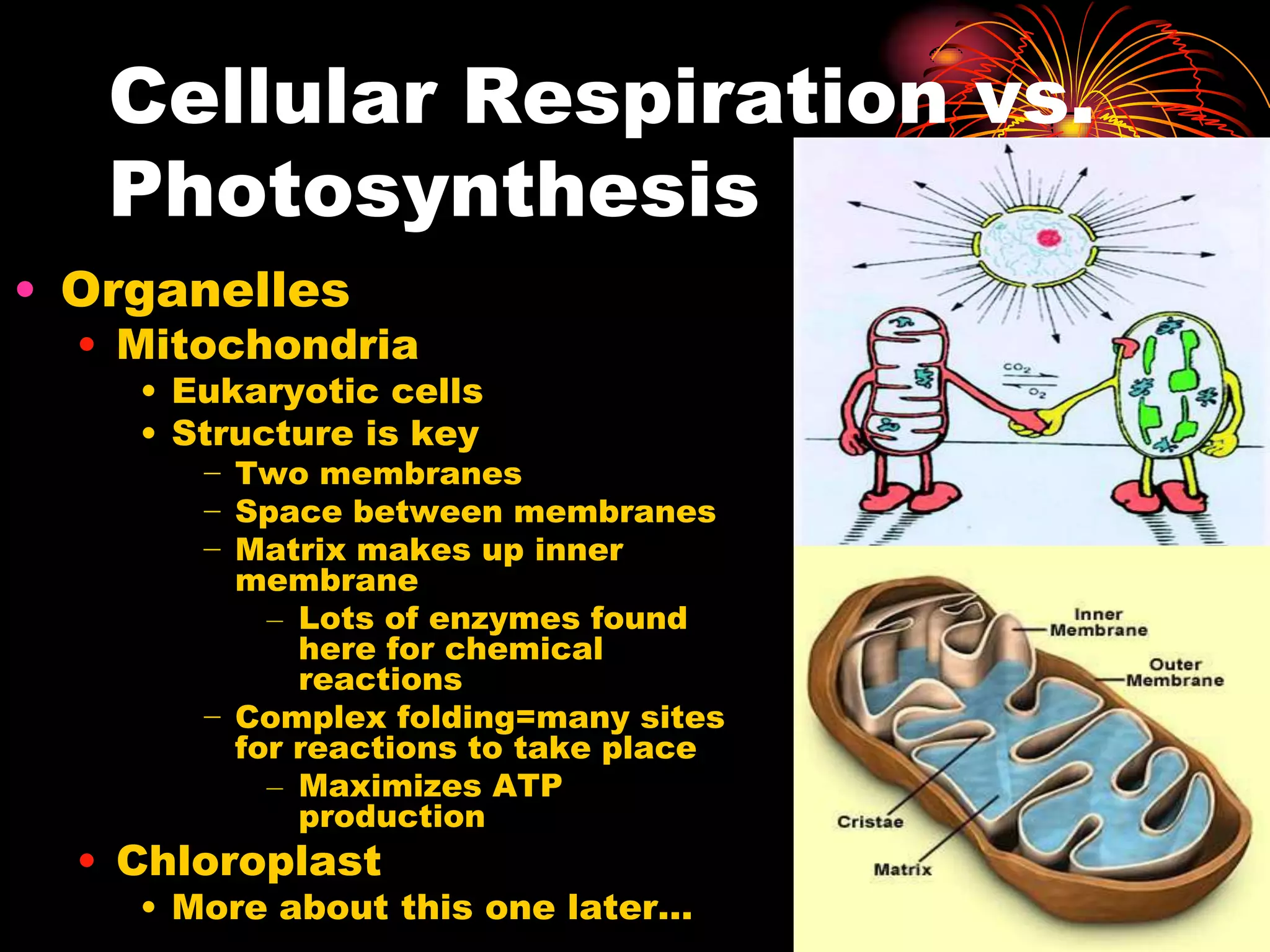
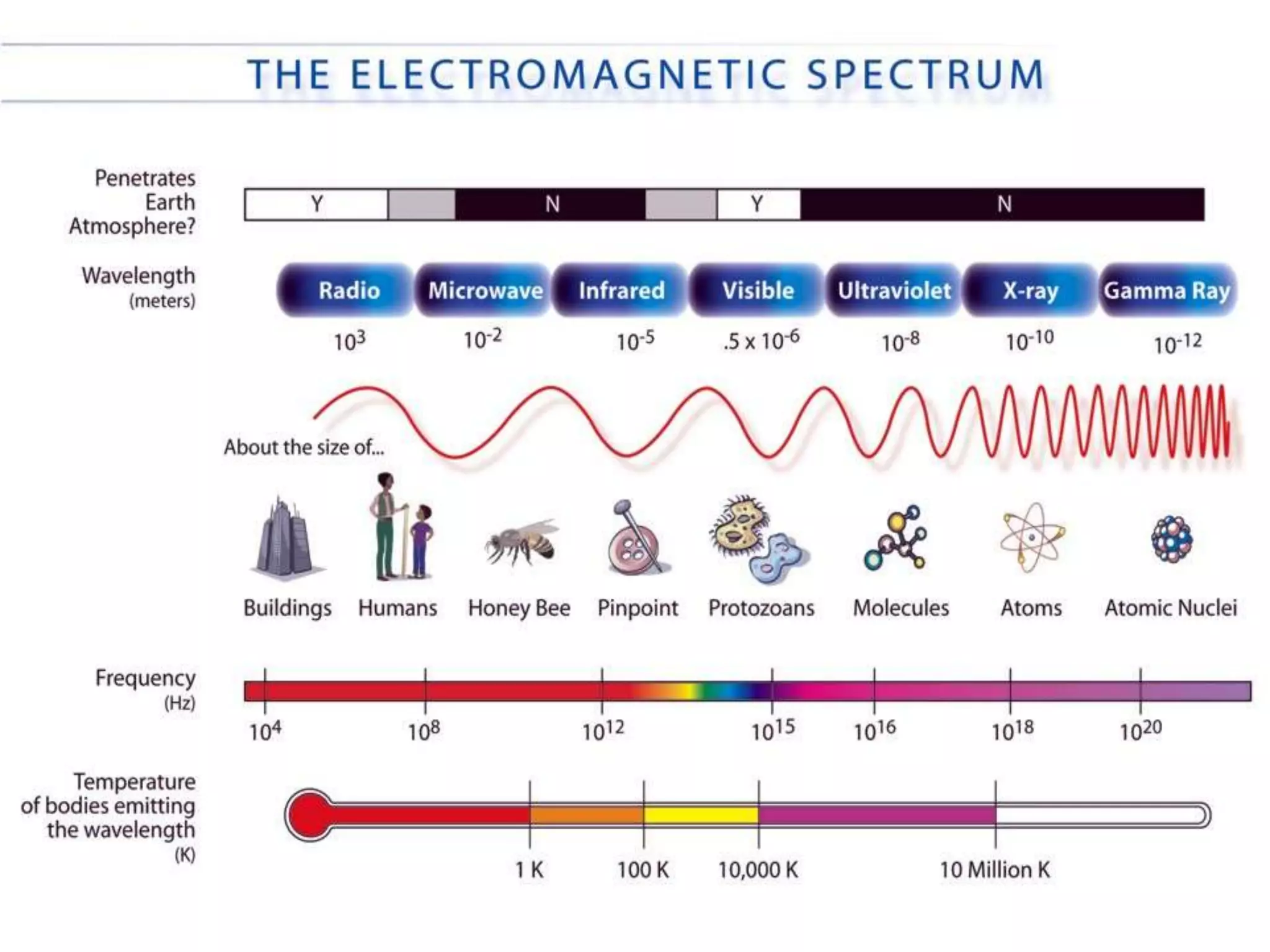
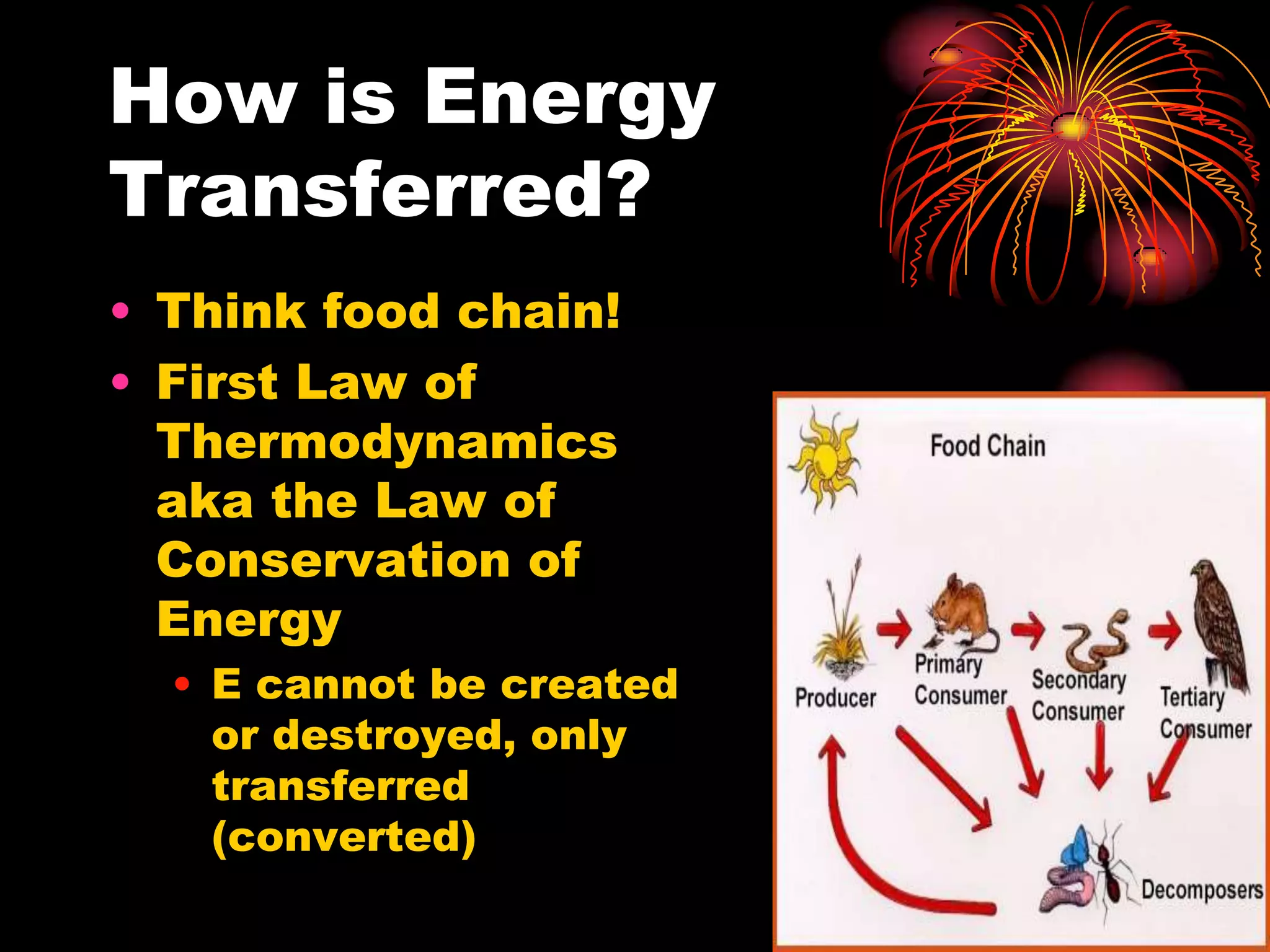
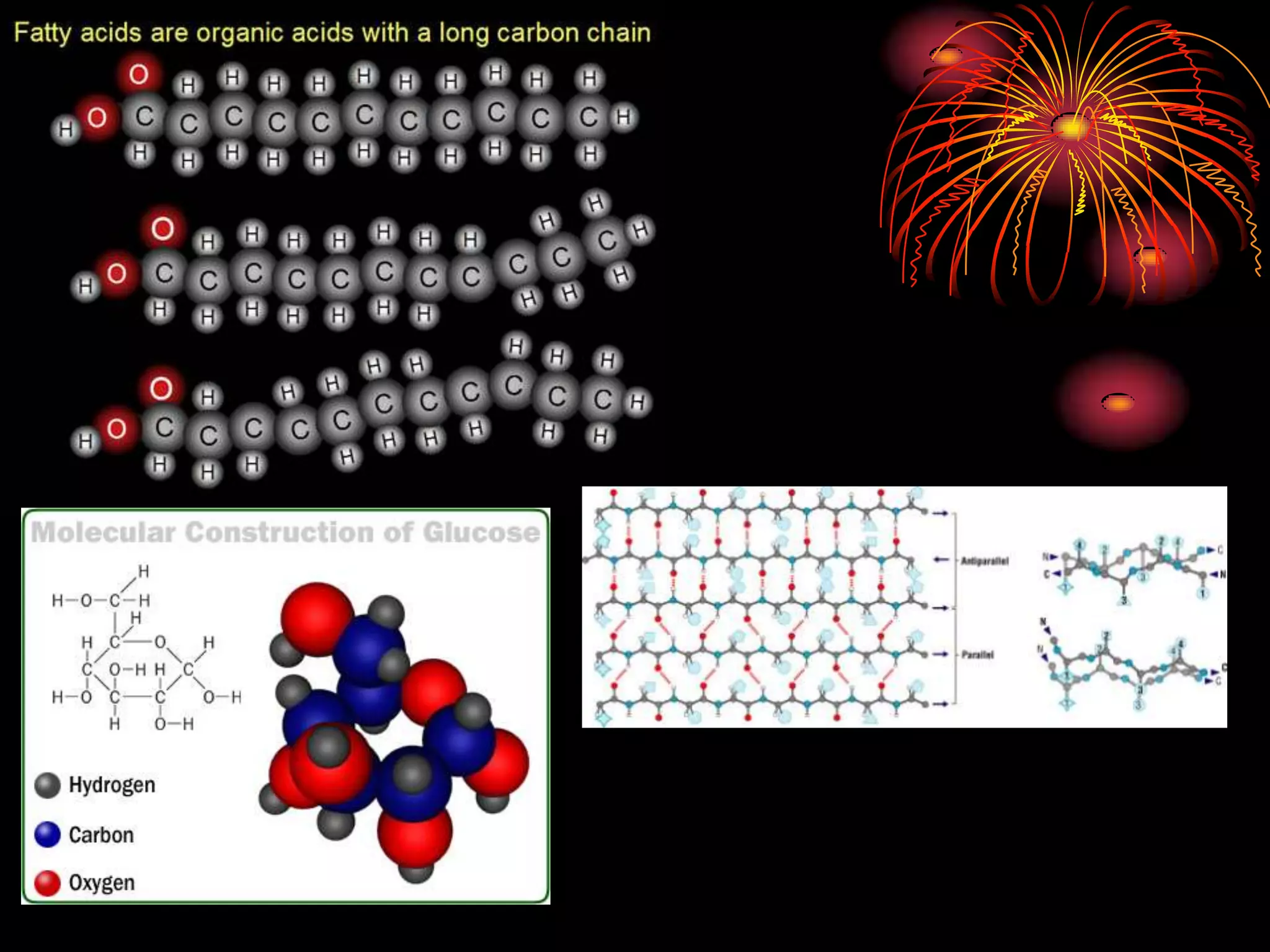
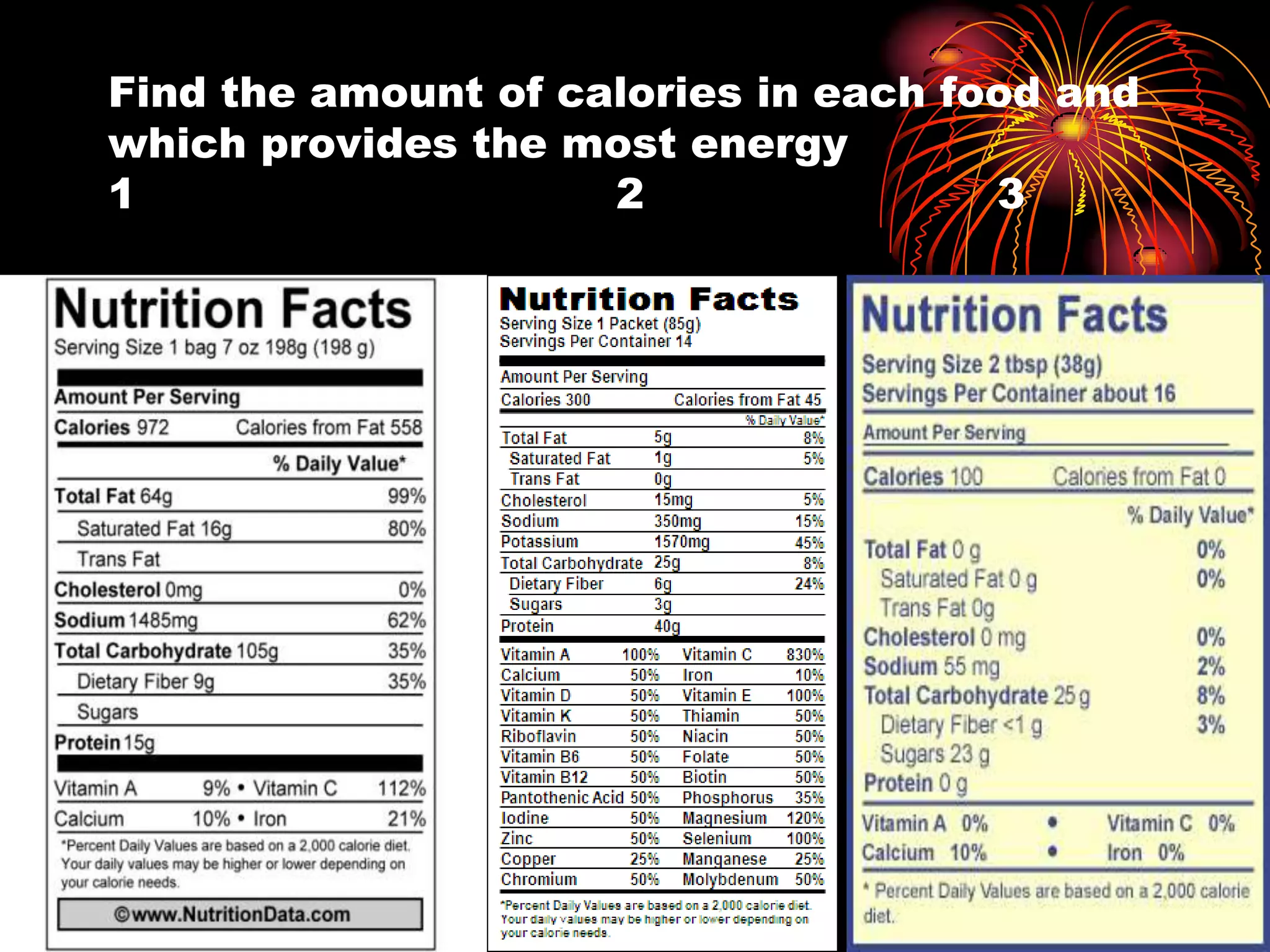
This document discusses cellular respiration and photosynthesis. It describes the structures and functions of mitochondria and chloroplasts. It explains how energy is transferred and the different forms it takes, including kinetic and potential energy. Chemical energy in foods like carbohydrates, fats, and proteins is broken down to release energy for cells to do work. Both cells and cars use combustion to break down glucose or hydrocarbons and release energy, producing carbon dioxide and water. Calories are a measurement of energy, with one calorie being the amount needed to raise 1 gram of water by 1 degree Celsius.