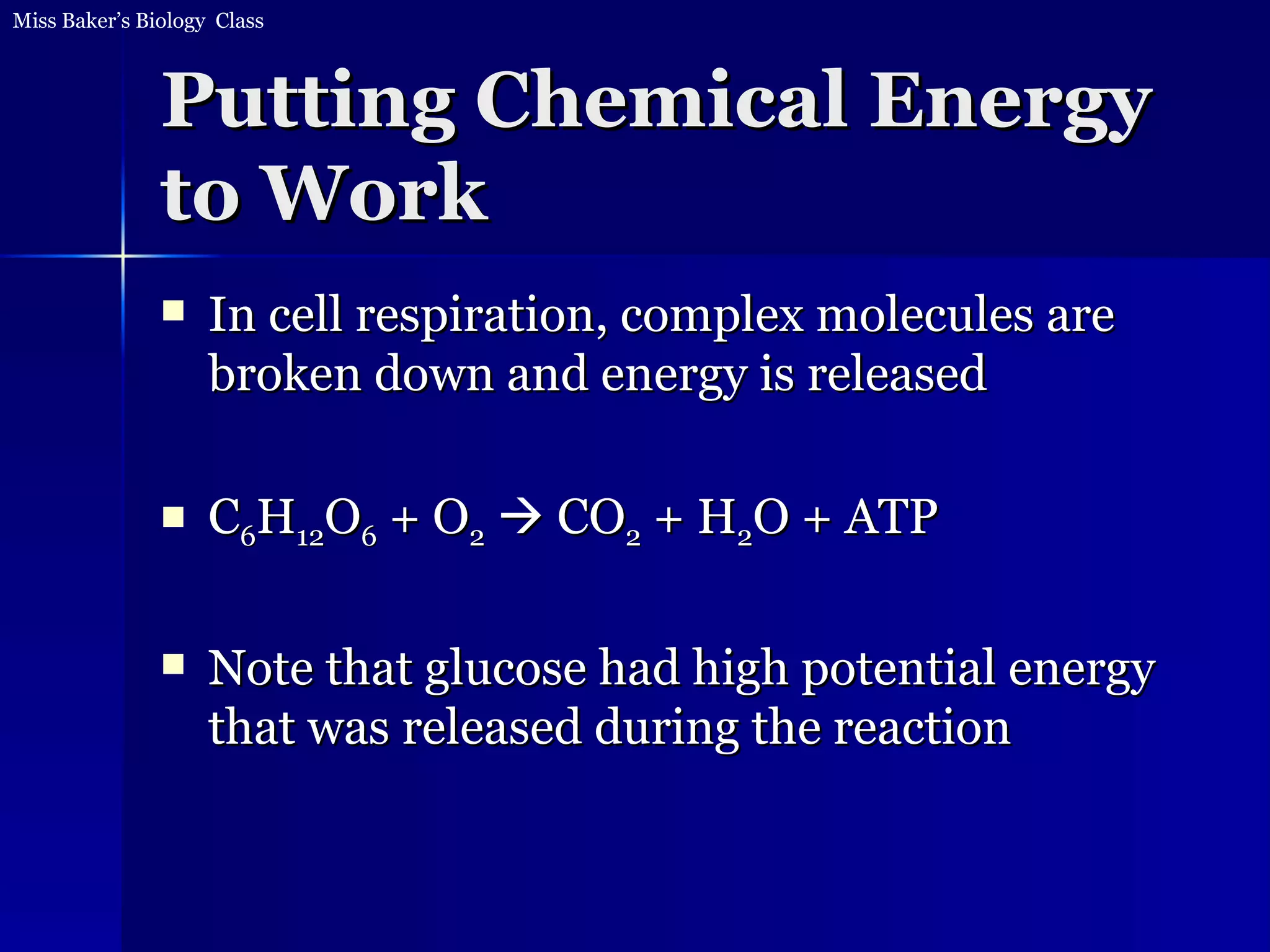
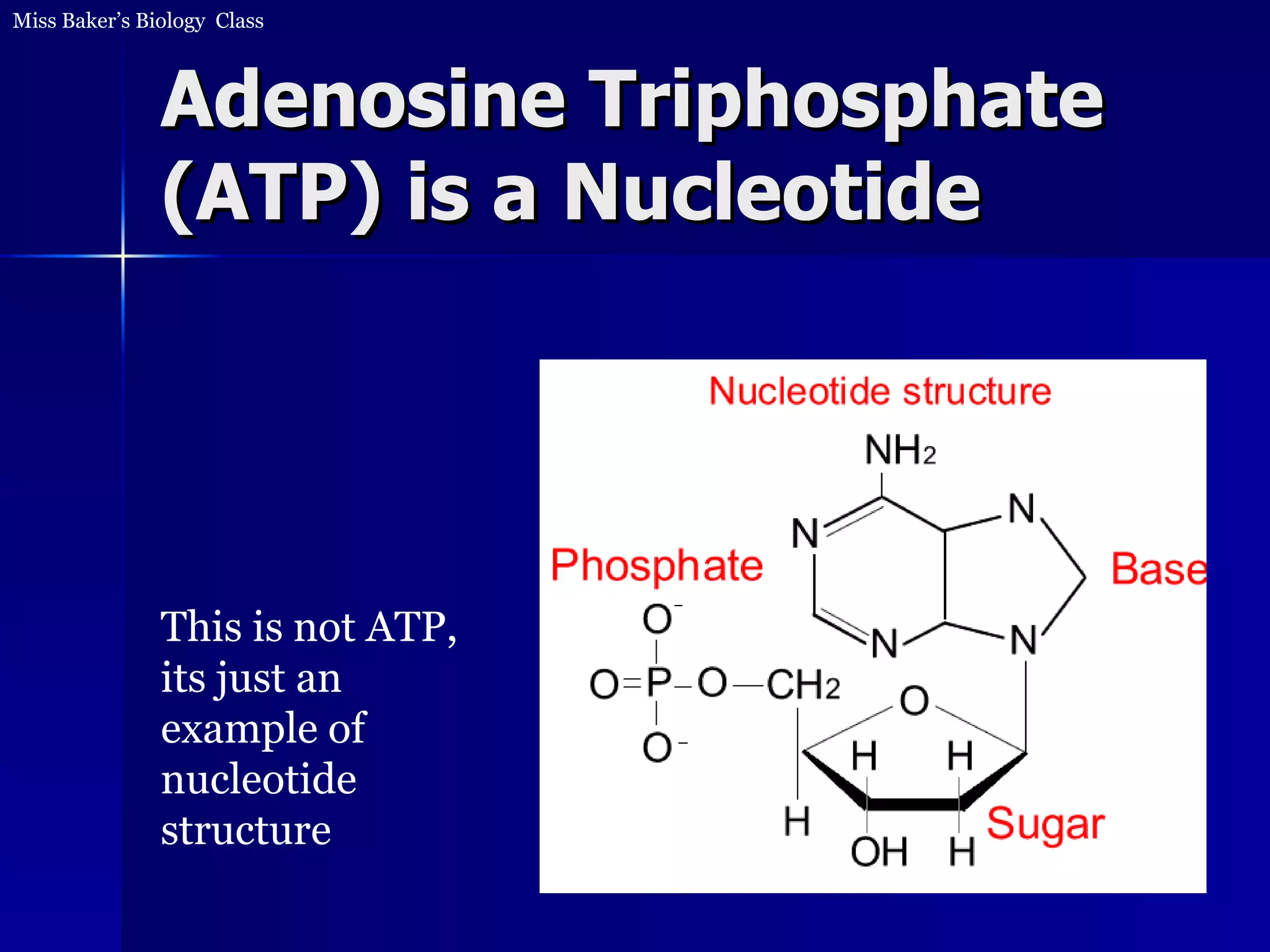
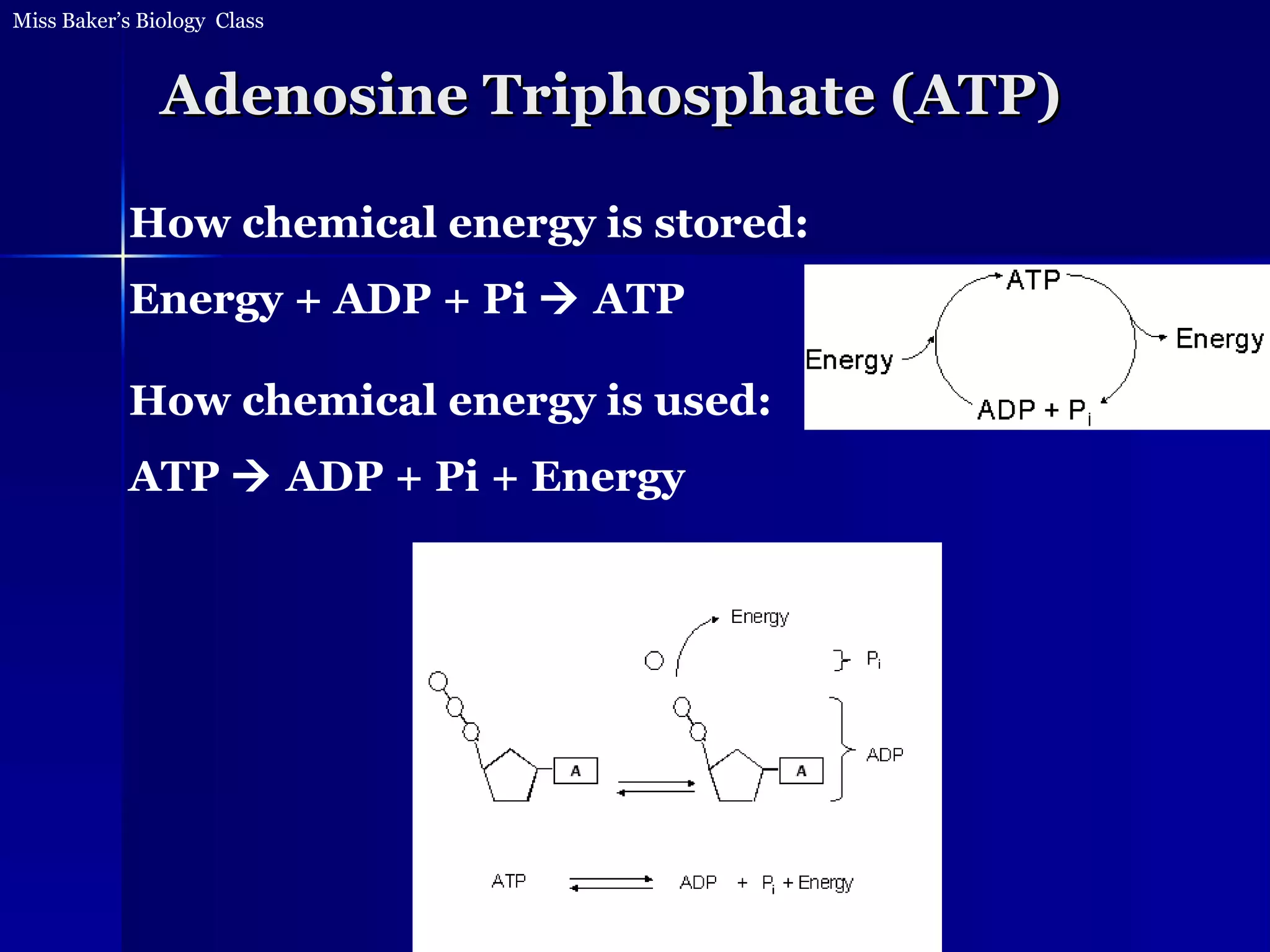
1. The document discusses how food provides chemical energy that cells can store and use. Chemical energy from glucose is stored in ATP through cell respiration and the breakdown of glucose.
2. ATP then provides chemical energy to drive other chemical reactions in cells. Whenever energy is converted, some is lost as heat and cannot be reused for other reactions.
3. The document defines calories as a unit of energy equal to the amount required to raise 1g of water by 1 degree C. Nutritional labels measure calories in kilocalories to account for the large number of calories in foods.