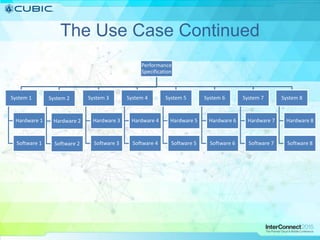
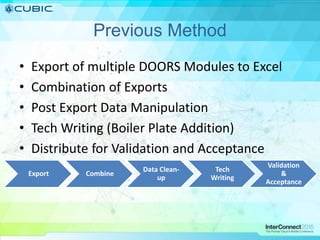
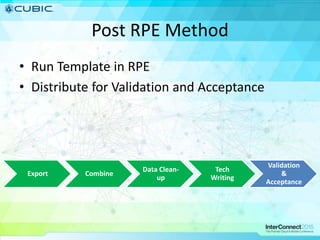
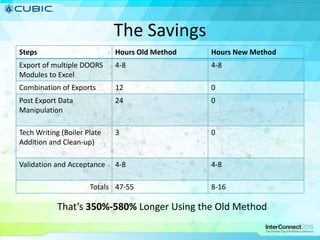
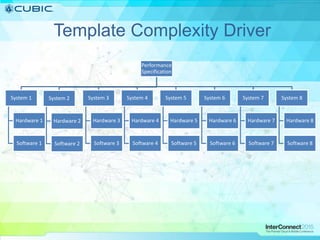
This document discusses how Cubic Simulation Systems uses DOORS and Rational Publishing Engine (RPE) to automate document production and save time. It describes a use case where Cubic had to produce 12 deliverables with multiple revisions for a customer. Using DOORS and RPE allowed Cubic to efficiently generate standardized documents, add/filter fields, and sort/filter reports to aid customer review while ensuring compliance. The system also maintains full traceability of document changes and impacts.