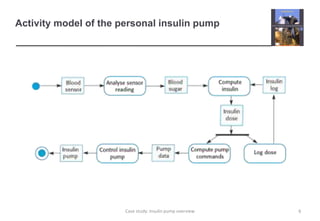
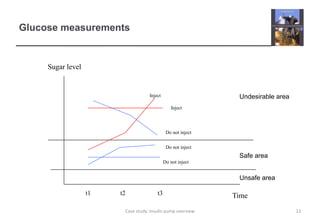
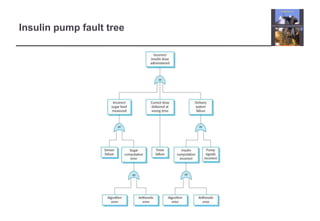
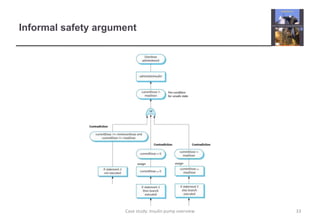
The document describes an insulin pump that measures a patient's blood sugar levels and automatically injects insulin to maintain safe levels. It functions by taking periodic glucose readings and comparing them to determine if insulin should be injected to counter rising sugar levels. The goal is to keep sugar within a safe band like a healthy pancreas would. The pump hardware, software requirements, and safety considerations are discussed to minimize risks like overdose or underdose from failures.