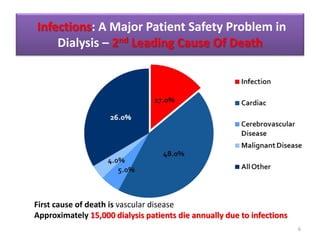
This document discusses infection control in dialysis units. It provides background on the high rates of infection in dialysis patients, who are immunosuppressed and undergo frequent medical procedures and hospitalizations. The second leading cause of death in dialysis patients is infection. The document then outlines strategies recommended by the CDC and other experts to reduce infection rates, including surveillance and feedback, hand hygiene, chlorhexidine use, catheter care guidelines, and staff education. Standard precautions like environmental cleaning and proper use of personal protective equipment are also emphasized.































![• Use standard cleaning and disinfection
protocols and EPA-registered hospital
disinfectants for confirmed or suspected
antibiotic-resistant Gram-positive cocci (e.g.,
MRSA, vancomycin intermediate–resistant S.
aureus, or vancomycin-resistant Enterococcus
[VRE]).](https://image.slidesharecdn.com/infectioncontrolwhyandhow-170120000237/85/Infection-control-why-and-how-35-320.jpg)