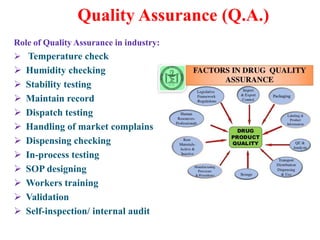
Kishan Patel completed an industrial training report at Swiss Garnier, a pharmaceutical manufacturer. Swiss Garnier has various departments including warehouse, production, quality control, quality assurance, and marketing. Kishan observed the tablet manufacturing process, which involves steps like material dispensing, granulation, milling, blending, compression, and quality testing. He also learned about the roles of quality control and quality assurance in ensuring proper raw material receipt, testing, and dispatch. The training helped enhance his academic knowledge and identify his strengths and weaknesses.