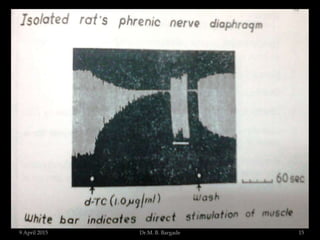
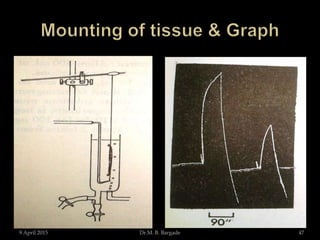
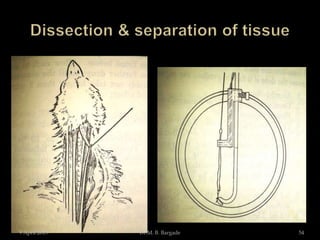
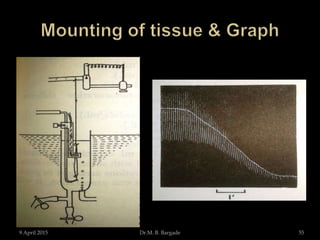
The document outlines pharmacological experiments focused on bioassays for evaluating drug effects on isolated muscle preparations. It details various methods and procedures for conducting assays, including the use of different animal tissues, concentrations of drugs, and the effects of various agonists and antagonists. The purpose of these experiments is to accurately estimate the potency and activity of pharmaceutical compounds using biological methods.











![ Effect of following drugs on preparations-
1] Ach 10-2 M
2]Decamethonium
6*10-3 M or
suxamethonium 2*10-
3 M
3] Hexamethonium
2*10-2 M
4] Tubocurarine 2*10-4 M or
Gallamine triethiodide 5*10-3
M
5] Tubocurarine or Gallamine
(as in 4) f/b 3 min after without
wash, 0.2 ml Physostigmine 10-4
M or neostigmine 10-4 M
9 April 2015 16Dr.M. B. Bargade](https://image.slidesharecdn.com/experimentsonisolatedskeletalmuscles-mukunda-150409023408-conversion-gate01/85/Experiments-on-isolated-skeletal-muscles-mukunda-16-320.jpg)
![6] Decamethonium 6*10-3
M or suxamethonium
2*10-3 M f/b 3 min after
without wash, 0.2 ml
Physostigmine 10-4 M or
neostigmine 10-4 M
7] 0.2 ml Physostigmine
10-4 M or neostigmine 10-4
M f/b 1min after without
wash O.2 ml Ach 10-2 M
8] 0.2 ml Lignocaine 3*10-2
M
9 April 2015 17Dr.M. B. Bargade](https://image.slidesharecdn.com/experimentsonisolatedskeletalmuscles-mukunda-150409023408-conversion-gate01/85/Experiments-on-isolated-skeletal-muscles-mukunda-17-320.jpg)


























![ Effect of following drugs on preparations-
1] Ach 10-2 M
2]Decamethonium
10-4 M or
suxamethonium 10-4
M
3] Hexamethonium
10-2 M
4] Tubocurarine 2*10-3 M or
Gallamine triethiodide 10-3
M
5] Tubocurarine or Gallamine
(as in 4) f/b 3 min after without
wash, 0.2 ml Physostigmine 10-4
M or neostigmine 10-4 M
9 April 2015 56Dr.M. B. Bargade](https://image.slidesharecdn.com/experimentsonisolatedskeletalmuscles-mukunda-150409023408-conversion-gate01/85/Experiments-on-isolated-skeletal-muscles-mukunda-56-320.jpg)
![6] Decamethonium 10-4 M
or suxamethonium 10-4 M,
f/b 3 min after without
wash, 0.2 ml
Physostigmine 10-4 M or
neostigmine 10-4 M
7] 0.2 ml Physostigmine
10-4 M or neostigmine 10-4
M f/b 1 min after without
wash O.2 ml Ach 10-2 M
8] 0.2 ml Lignocaine 3*10-2
M
9 April 2015 57Dr.M. B. Bargade](https://image.slidesharecdn.com/experimentsonisolatedskeletalmuscles-mukunda-150409023408-conversion-gate01/85/Experiments-on-isolated-skeletal-muscles-mukunda-57-320.jpg)







