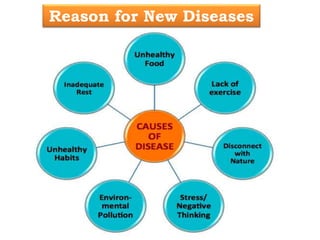
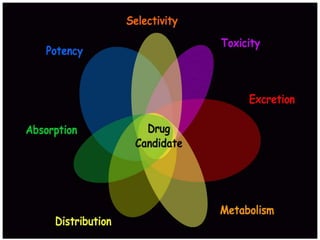
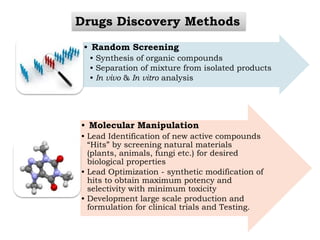
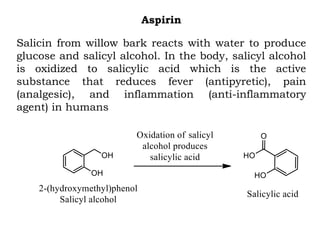
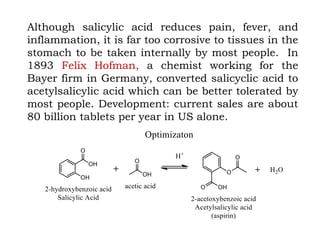
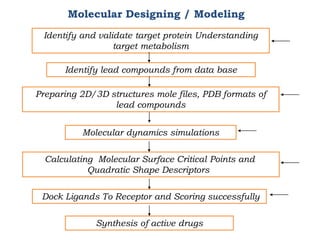
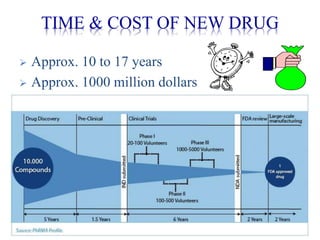
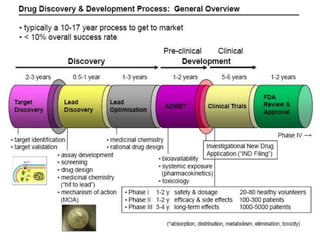
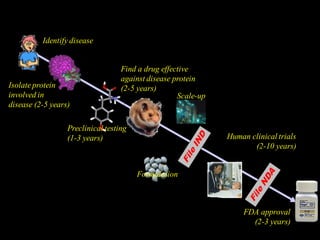
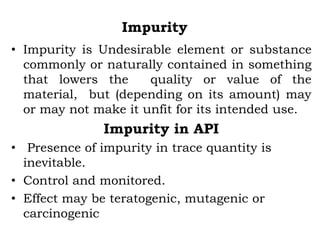
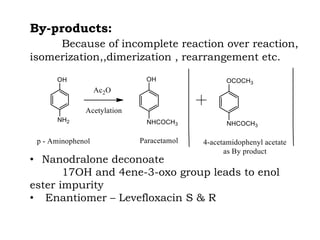
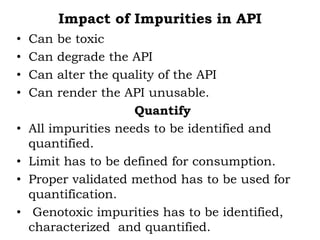
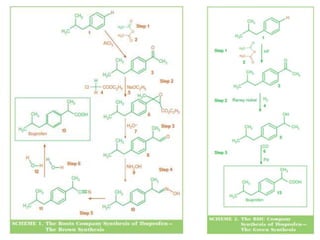
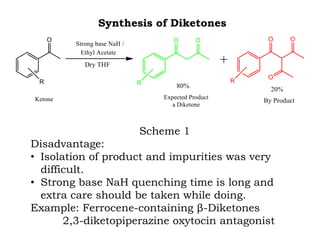
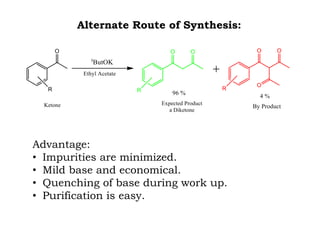
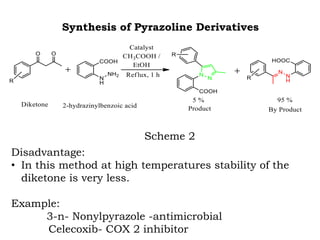
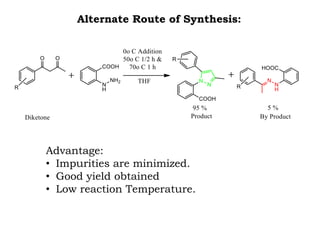
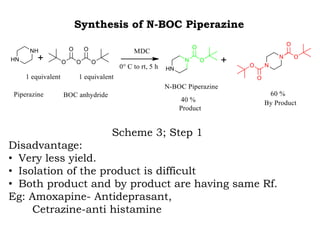
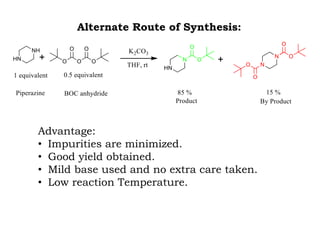
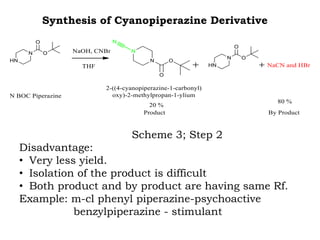
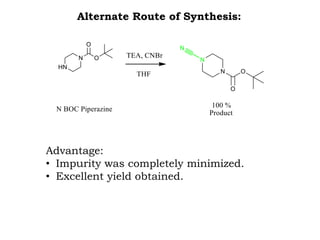
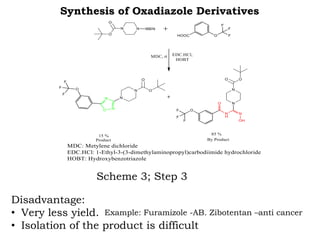
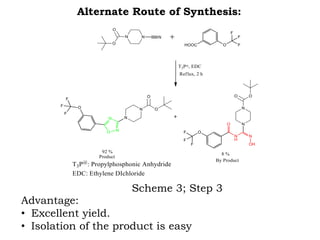
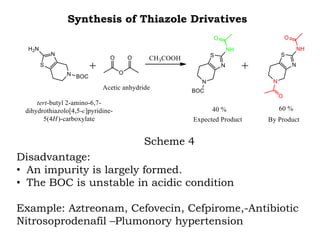
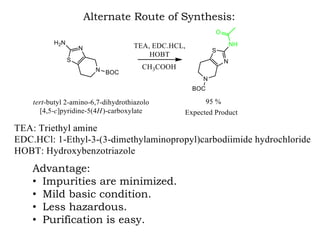
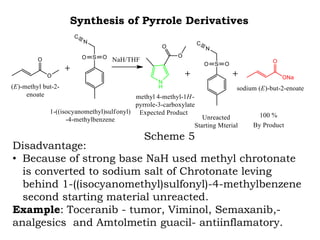
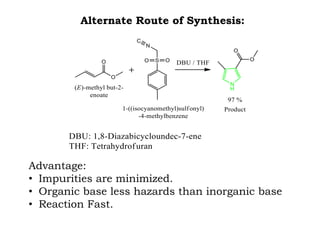
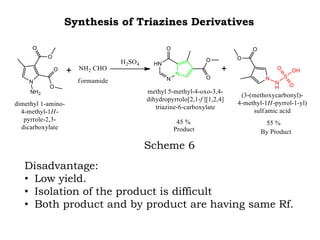
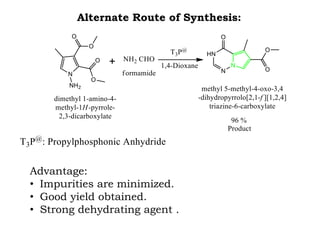
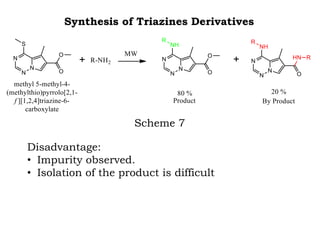
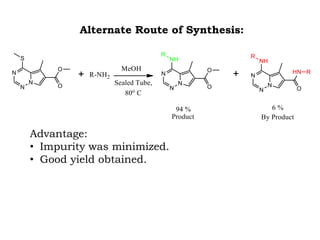
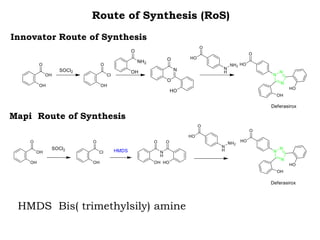
The document discusses the challenges and methodologies in drug synthesis, emphasizing the need for new drugs to combat emerging diseases and the complexities of ensuring drug purity. It covers various types of impurities found in active pharmaceutical ingredients (APIs), their impact, and strategies for purification and synthesis of different compounds. The collaborative effort of various scientific disciplines in pharmaceutical research and development is highlighted as essential in overcoming the time and cost hurdles of drug discovery.