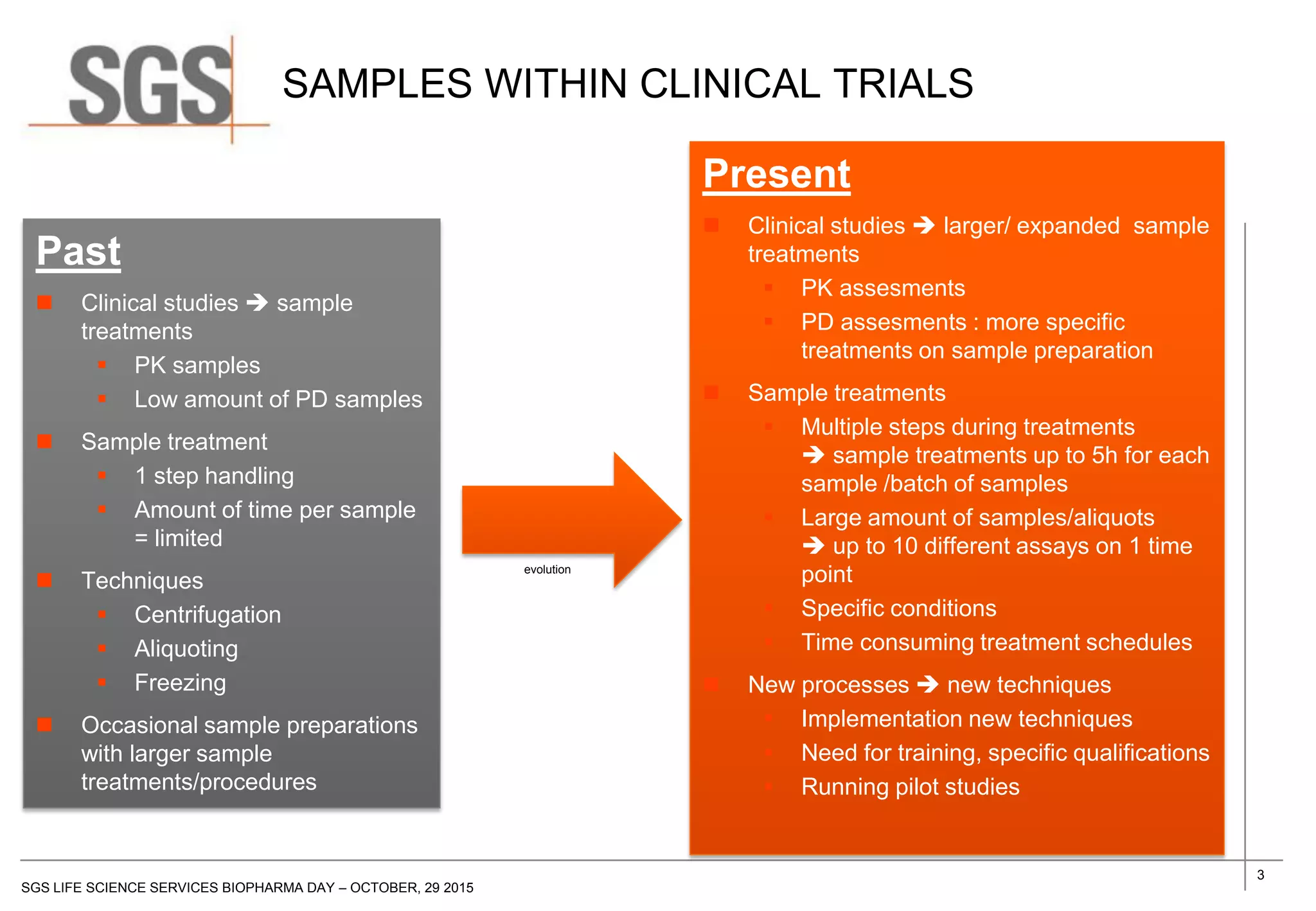
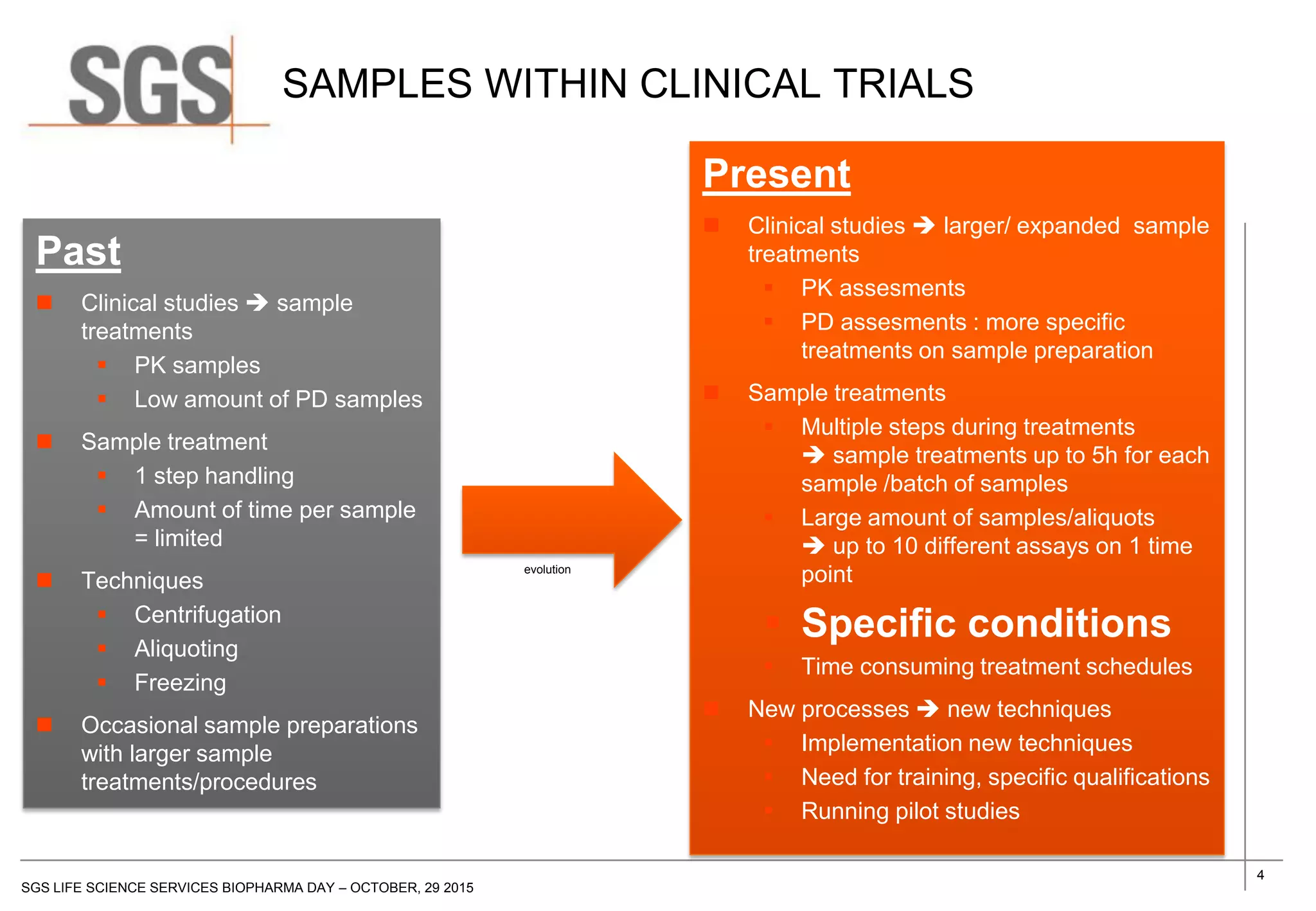
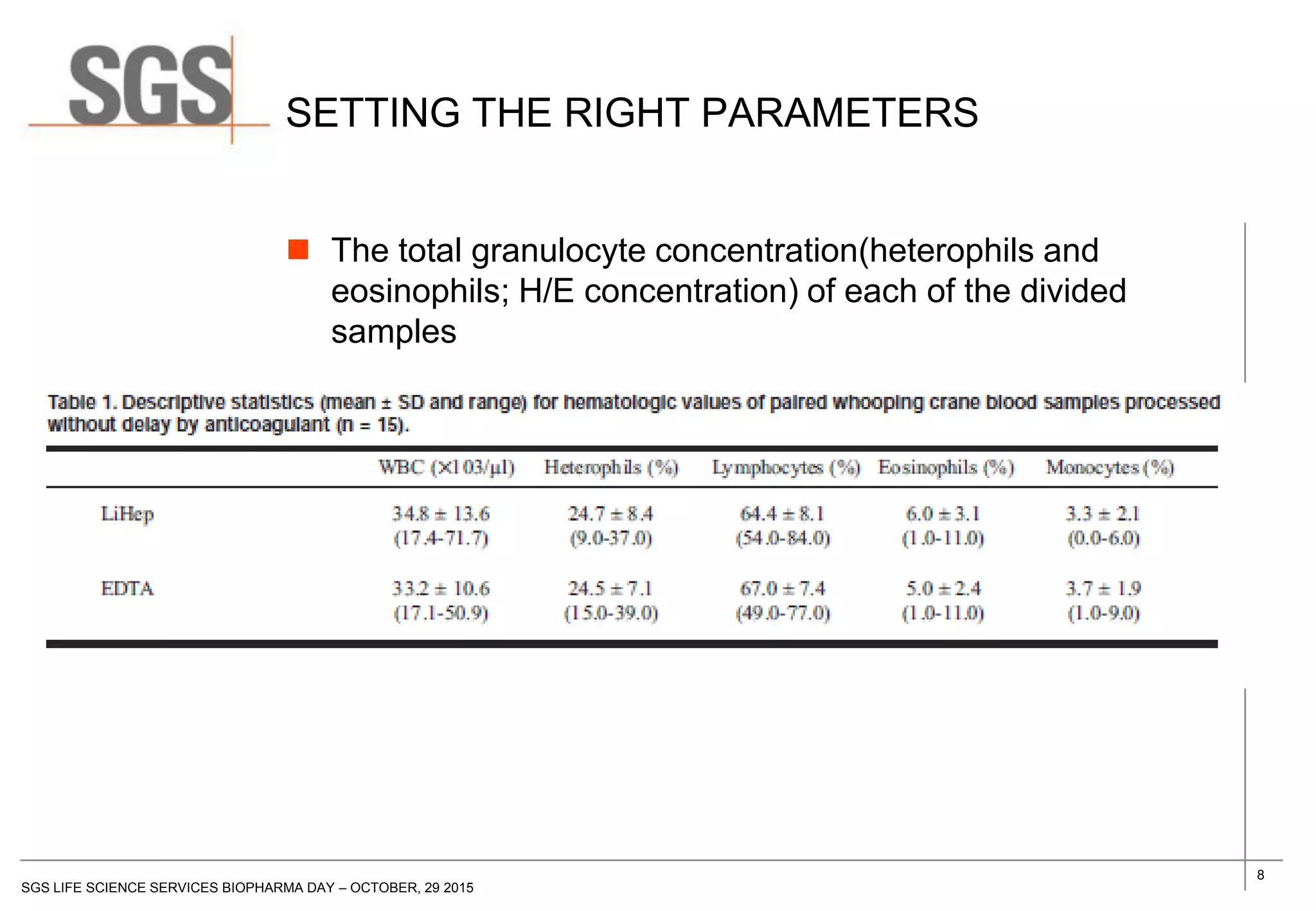
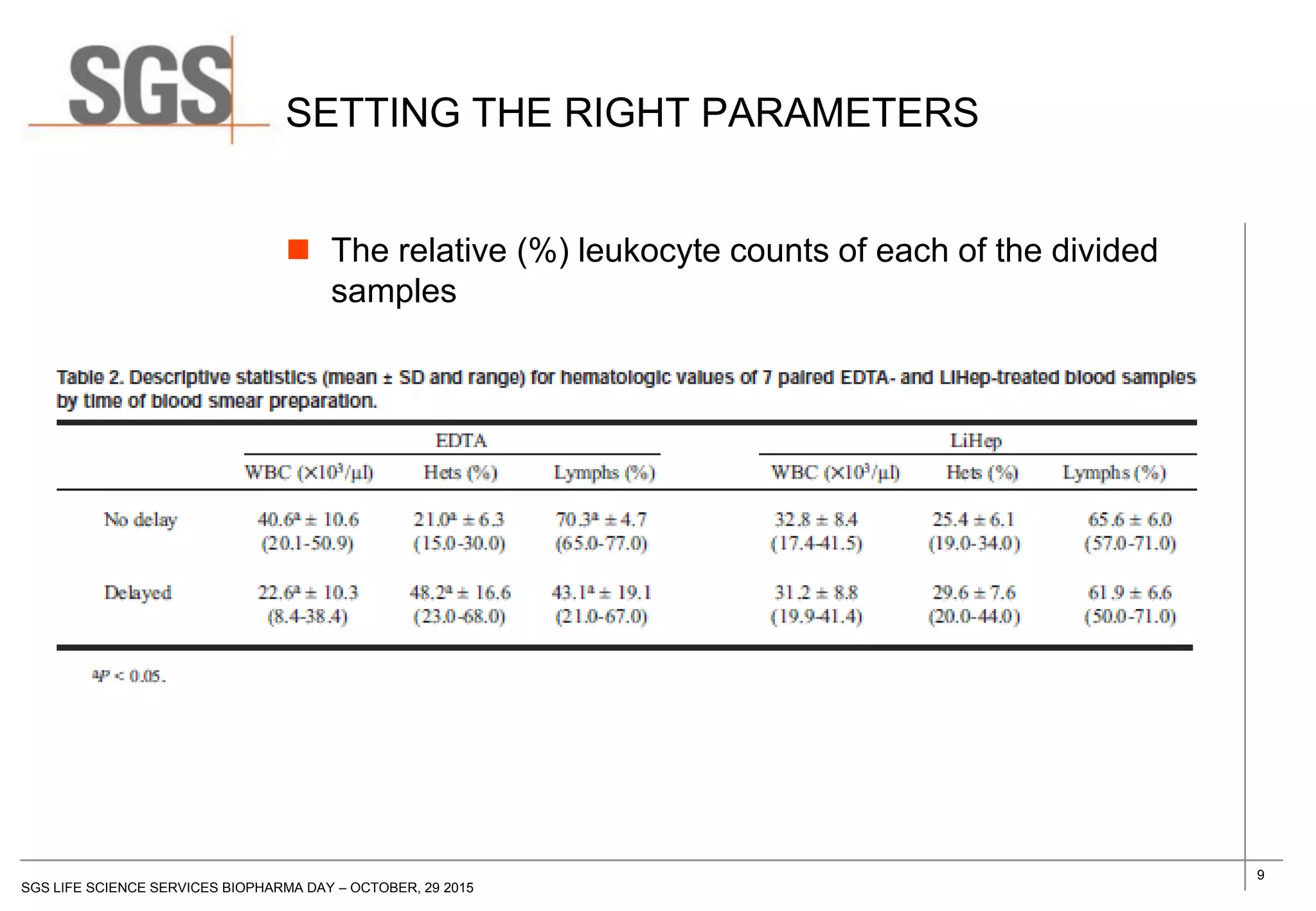
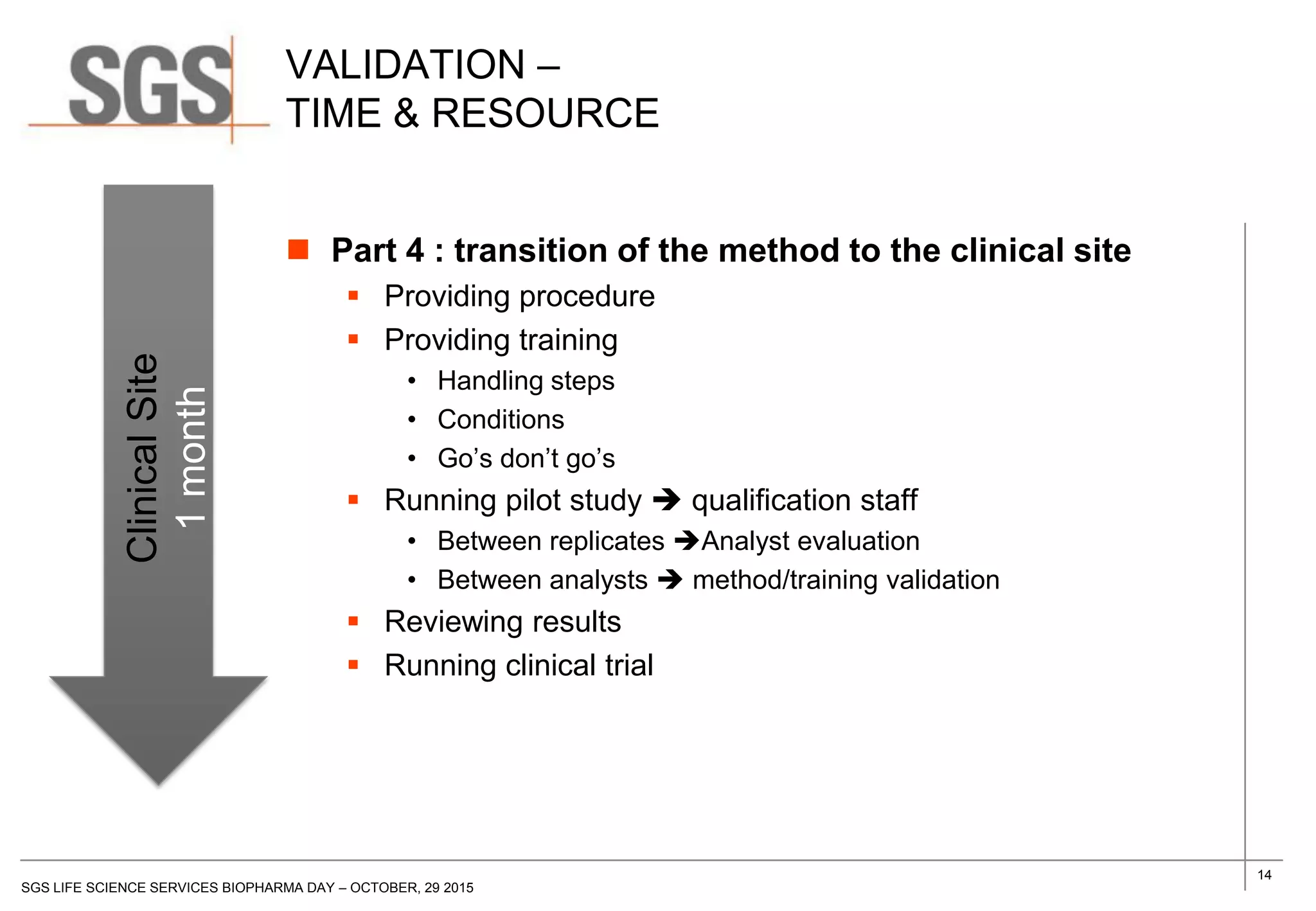
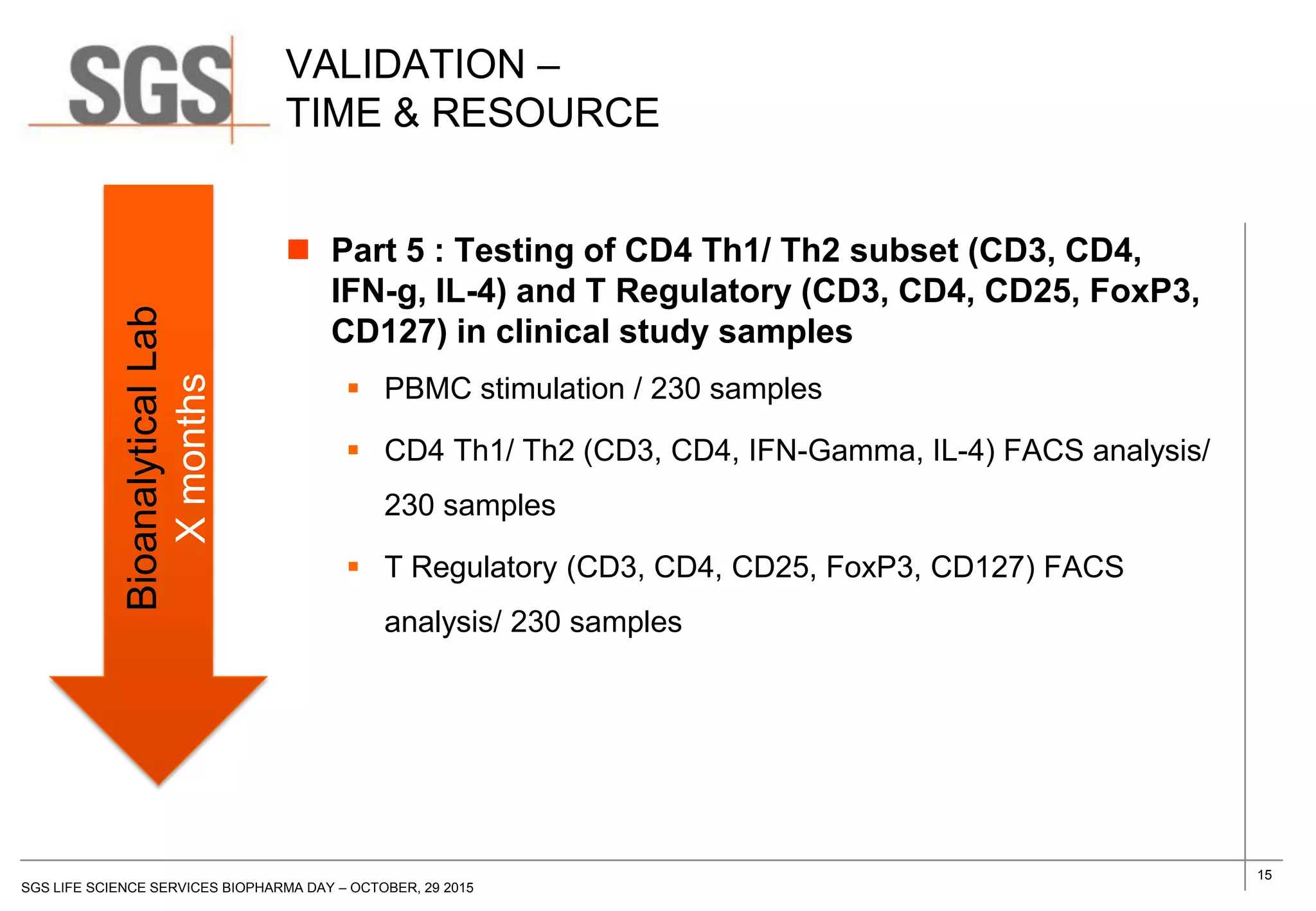
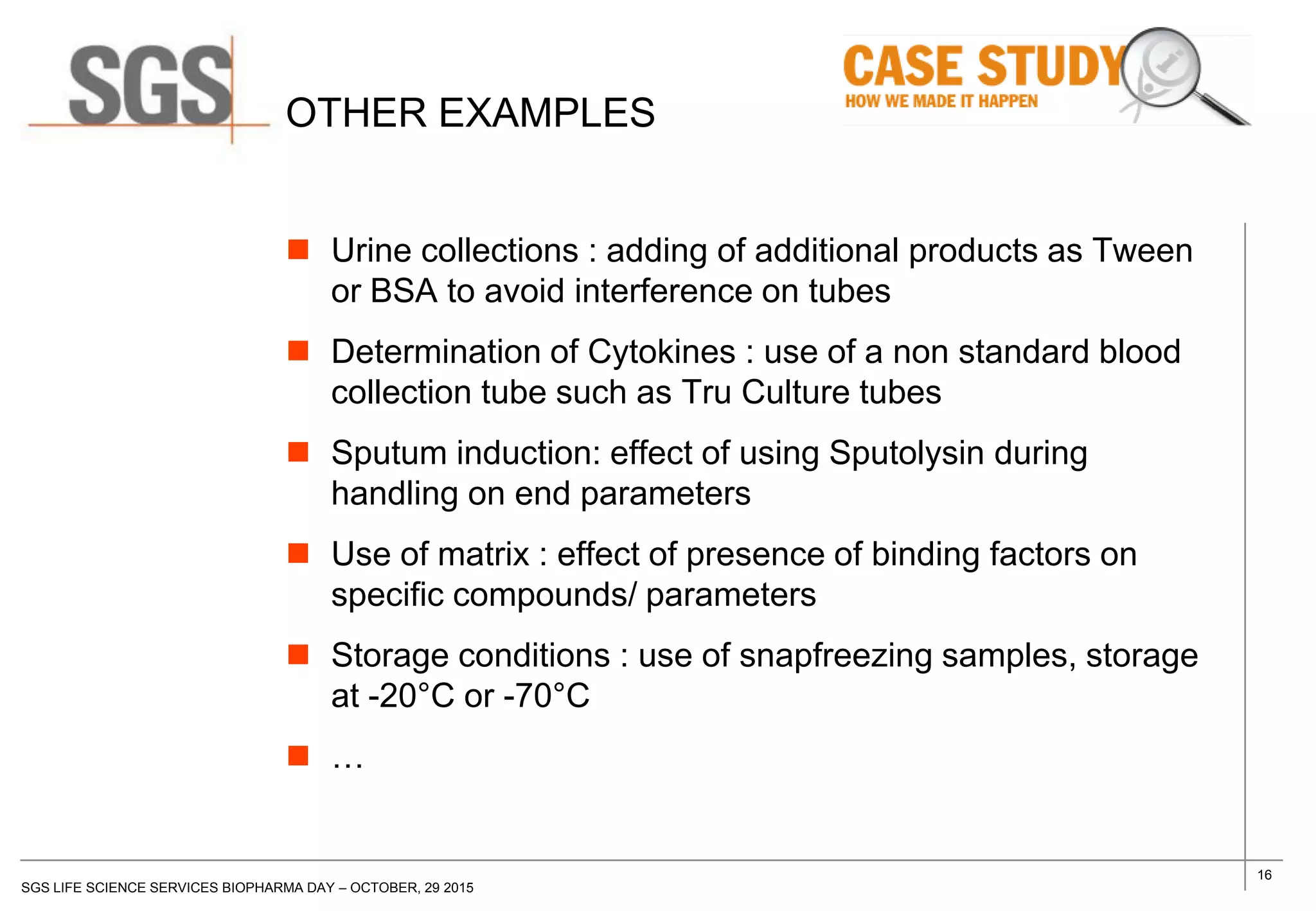
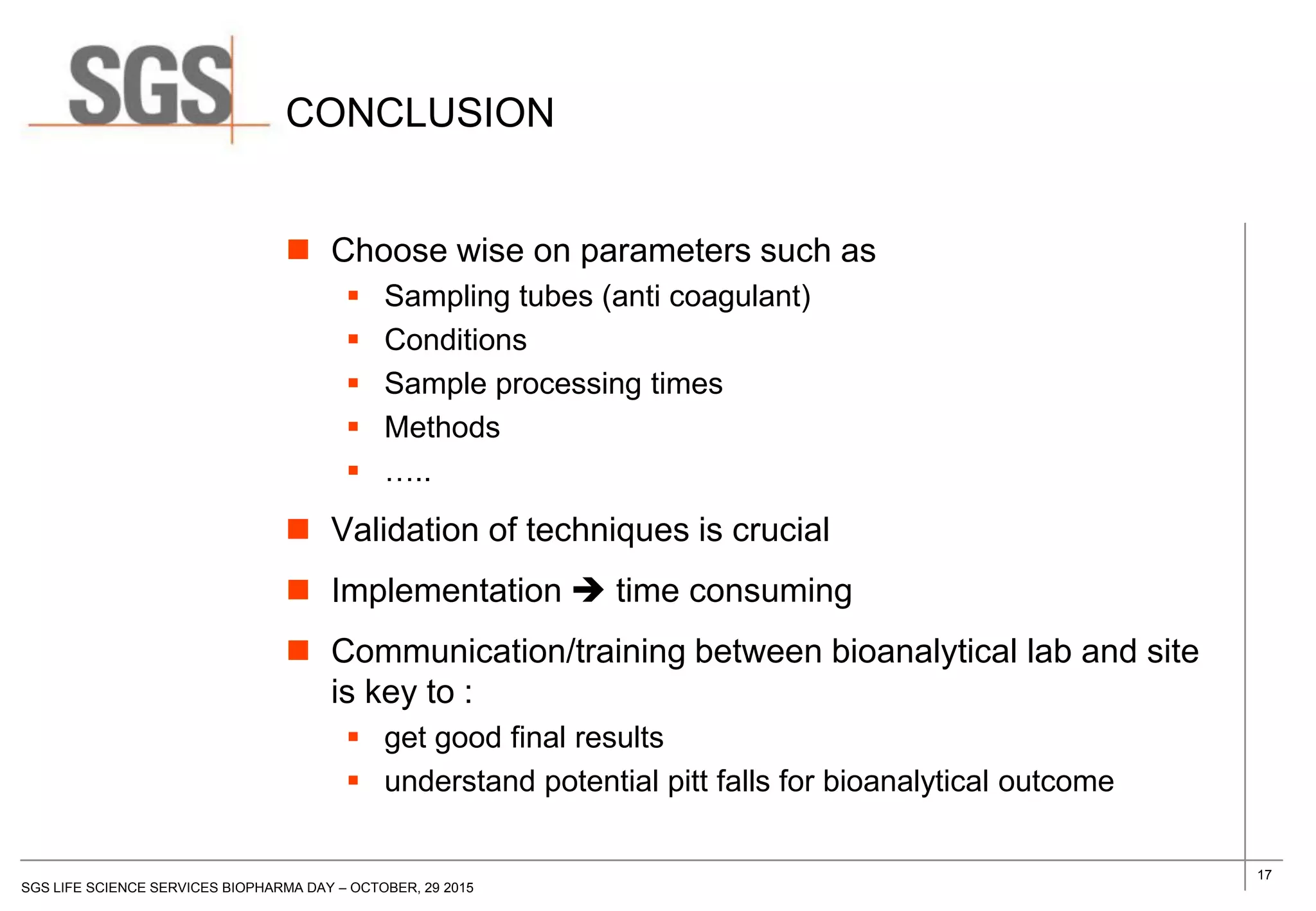
The document discusses the impact of sample handling and processing on bioanalytical outcomes in clinical trials, emphasizing that proper measures from sample collection to laboratory testing are essential due to the complexity and resource demands involved. It highlights the importance of validating techniques and implementing appropriate sampling conditions to ensure accurate results. Moreover, it underscores the need for effective communication and training between bioanalytical labs and clinical sites to avoid pitfalls in the process.