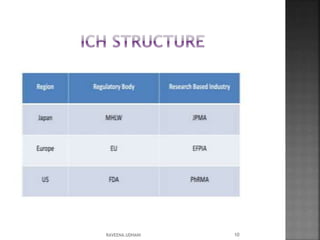
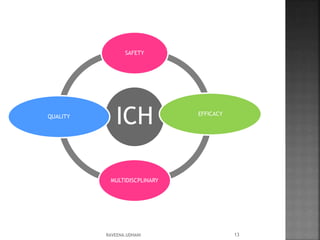
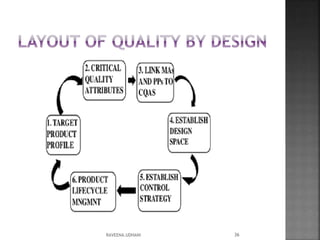
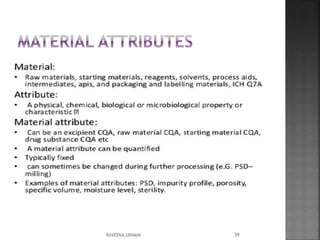
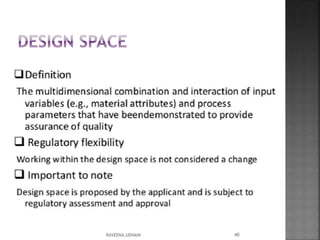
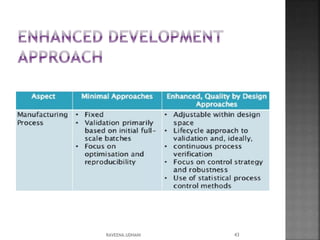
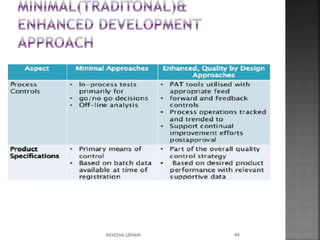
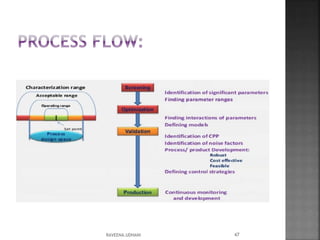
The document discusses the International Conference on Harmonisation (ICH), which aims to harmonize technical requirements for pharmaceutical registration internationally. It outlines ICH's objectives like reducing duplication of testing and making guidelines consistent. ICH has members from regulatory authorities and the pharmaceutical industry in the EU, Japan and US. The document also describes ICH's structure and working groups. Finally, it discusses ICH Q8 guidelines on pharmaceutical development and quality-by-design approach to building quality into products.