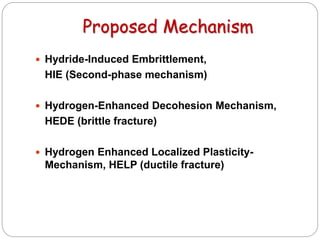
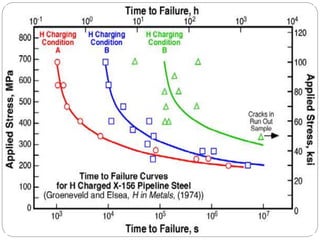
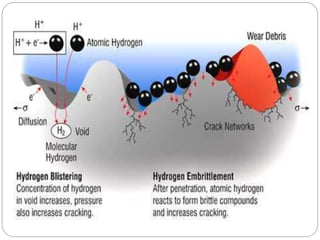
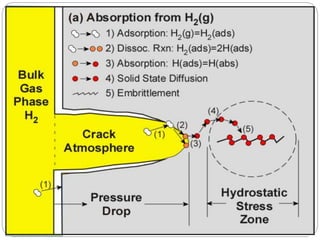
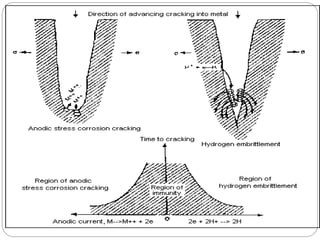
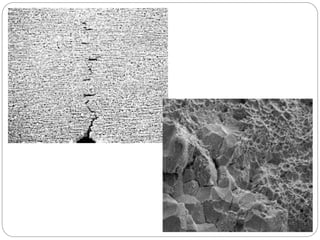
This document discusses hydrogen embrittlement, which is the loss of ductility and increased brittleness in metals caused by hydrogen absorption. It can occur during processes like corrosion and welding. BCC and HCP metals are more susceptible than FCC metals. The document covers the causes of hydrogen embrittlement including hydrogen introduction during melting/solidification and corrosion. It also discusses the mechanisms like hydride formation and hydrogen enhancing localized plasticity. Finally, it lists techniques to prevent hydrogen embrittlement such as reducing corrosion, using clean steel, baking, proper welding techniques, and alloying with nickel or molybdenum.



![Hydrogen may be introduced during :
[1] During Melting & Entrapped during
Solidification,
[2] Anodic Reaction during Corrosion,
[3] Hydrogen Gas Welding & Moistured
Electrode](https://image.slidesharecdn.com/hydrogen-110416120206-phpapp02hydrogenembrittlement-150723012432-lva1-app6892/85/Hydrogen-110416120206-phpapp02-hydrogen-embrittlement-4-320.jpg)
![The Chief characteristics of Hydrogen
Embrittlement :
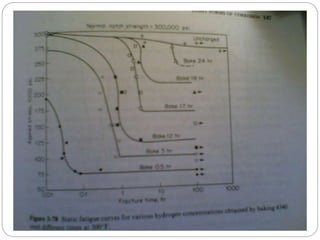
[1] Strain Rate Sensitivity increases,
[2] Susceptibility to Delayed Fracture
increases.
Hydrogen Embrittlement is enhanced by slow strain rates.
At low temperatures and high temperatures hydrogen
embrittlement is negligible, but it is most severe at Room
Temperature for example steel. Slow bend test and
Notched and Unnotched tension tests will detect hydrogen
Embrittlement by a drastic decrease in ductility, but](https://image.slidesharecdn.com/hydrogen-110416120206-phpapp02hydrogenembrittlement-150723012432-lva1-app6892/85/Hydrogen-110416120206-phpapp02-hydrogen-embrittlement-5-320.jpg)








![References
[1] George E. Dieter and David Bacon, “Mechanical Metallurgy,”
McGraw-Hill Book Company, ISBN 0-07-100406-8.
[2] Mar G. Fontana, “Corrosion Engineering,” McGraw-Hill Book
Company, ISBN 978-0-07-060744-6.
[3] G. Alefeld and J. Volkl (eds.), "Hydrogen in Metals,“ Springer-
Verlag, Berlin, Vols. 1 and 2, 1978.
[4] R. Kirchheim, E. Fromm, E. Wicke, eds., Verlag, Munchen,
"Metals-Hydrogen Systems," 1989.
[5] A.M. Brass, J. Chêne, “Hydrogen Uptake in 316L Stainless
Steel: Conseq- uences on Tensile Properties,” Corrosion
Science 48 (2006) 3222-3242.
[6] C. L. Briant and S. K. Banerji (eds.), “Embrittlement of
Engineering Alloys,” Academic Press , New York, 1983.
[7] R. Gibala, R. F. Hehnemann (eds.), “Hydrogen Embrittlement
and Stress Corrosion Cracking,” American Society for Metals,
Metals Park, Ohio, 1984.](https://image.slidesharecdn.com/hydrogen-110416120206-phpapp02hydrogenembrittlement-150723012432-lva1-app6892/85/Hydrogen-110416120206-phpapp02-hydrogen-embrittlement-22-320.jpg)
![Cont..
[8] I. M. Bernstein and A. W. Thompson (eds.), “Hydrogen in
Metals,” Amer Soc. For Metals, Metals Park Ohio, 1974.
[9] L . W. Tsay, T. Y. Yang, “Reduction of hydrogen
embrittlement in an ultra-high-strength steel by laser surface
annealing,” Fatigue Fract Engng Mater Struct, 23, p325–333.
[10] H. Kamoutsi, G.N. Haidemenopoulos, V. Bontozoglou, S.
Pantelakis, “Corrosion-induced hydrogen embrittlement in
aluminum alloy 2024,” Corrosion Science, 48 (2006), p1209–
1224.
[11] R.A. Siddiqui, H.A. Abdullah, “Hydrogen embrittlement in
0.31% carbon steel used for petrochemical applications”,
Journal of Materials Processing Technology, 170 (2005) ,p430–
435.
[12] C. Pan, Y.J. Su, W.Y. Chu, et al, “Hydrogen embrittlement of
weld metal of austenitic stainless steels”, Corrosion Science,
44 (2002), p1983.
[13] P. Sofronis, I.M. Robertson, “Viable Mechanisms of
Hydrogen embrittlement-A Review,” American Institute of](https://image.slidesharecdn.com/hydrogen-110416120206-phpapp02hydrogenembrittlement-150723012432-lva1-app6892/85/Hydrogen-110416120206-phpapp02-hydrogen-embrittlement-23-320.jpg)
