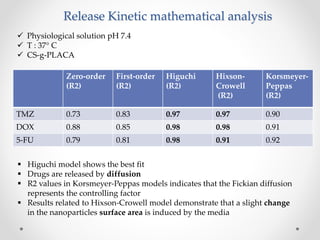
This document discusses the preparation and characterization of chitosan grafted carboxy functionalized polylactide nanoparticles for controlled and sustained release of multiple drugs including doxorubicin, temozolomide, and 5-fluorouracil. It highlights the advantages of using polysaccharide-based nanoparticles in drug delivery systems for enhanced efficacy and reduced side effects. The results indicate high encapsulation efficiency, stability, and a balanced release profile of the drugs at physiological conditions.