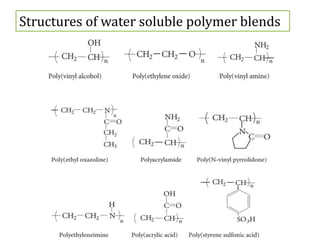
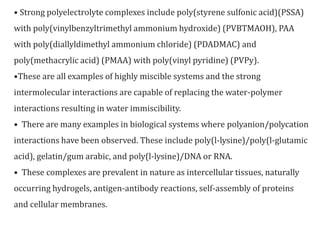
This document discusses water soluble polymer blends. It notes that many water soluble polymers can exhibit miscibility when blended with non-water soluble polymers. Examples of water soluble polymers that can form blends include poly(ethylene oxide), poly(acrylic acid), poly(vinyl alcohol), and poly(vinyl pyrrolidone). Strong interactions between acidic and basic water soluble polymers can lead to complexes that are insoluble in water. Examples of complexes include poly(styrene sulfonic acid) with poly(vinylbenzyltrimethyl ammonium hydroxide) and poly(acrylic acid) with poly(vinyl pyridine). Characterization techniques like conductometry can be used to determine the stoichi