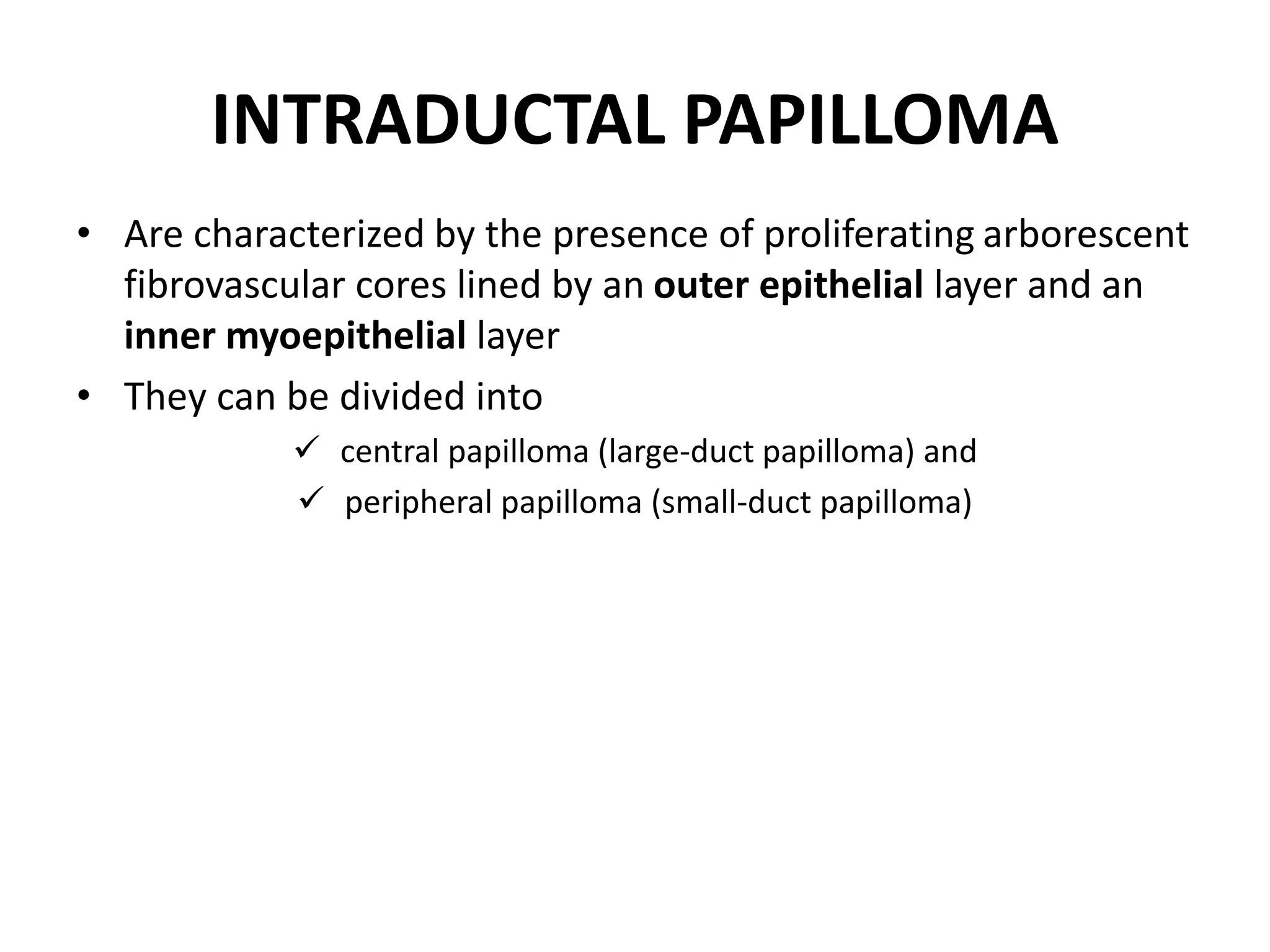
Papillary lesions of the breast form a spectrum ranging from benign intraductal papillomas to preinvasive and invasive papillary carcinomas. Intraductal papillomas are characterized by arborescent fibrovascular cores lined by epithelial cells and can be central or peripheral in location. Some papillomas may have areas of atypical ductal hyperplasia or ductal carcinoma in situ. Intraductal papillary carcinoma lacks a myoepithelial cell layer. Encapsulated papillary carcinoma and solid papillary carcinoma are rare variants that typically have a good prognosis with local therapy. Invasive papillary carcinoma is exceedingly rare.