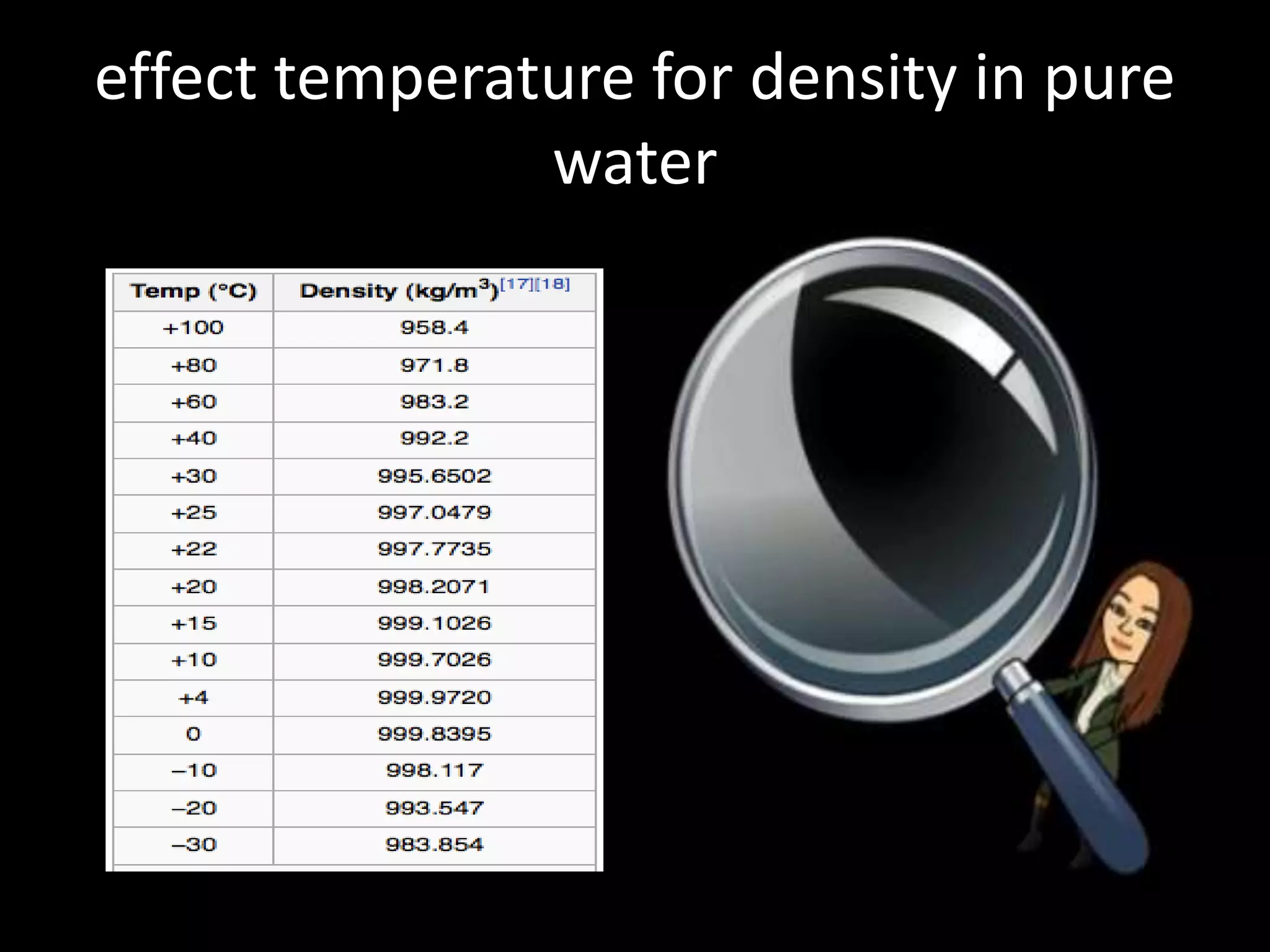
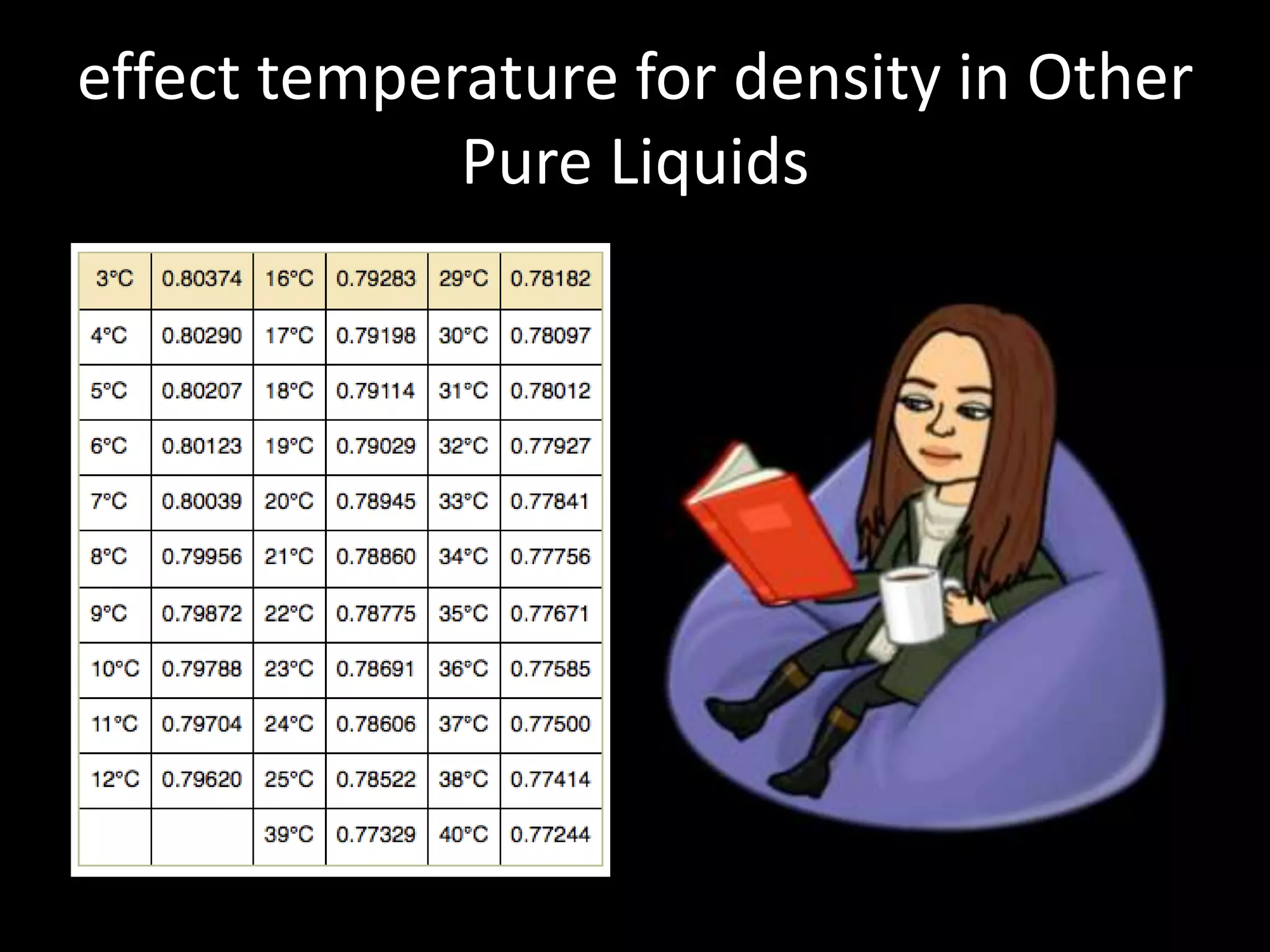
This document discusses how temperature affects density. It explains that as temperature increases, the kinetic energy of particles in a substance increases, causing the particles to move faster and reducing the density. Temperature can cause substances to change phases from solid to liquid to gas, expanding their volume and decreasing density. The document provides examples showing that gases have lower density than liquids or solids because temperature increases their kinetic energy and volume. It concludes that temperature directly impacts a substance's density by changing the energy and movement of its particles.