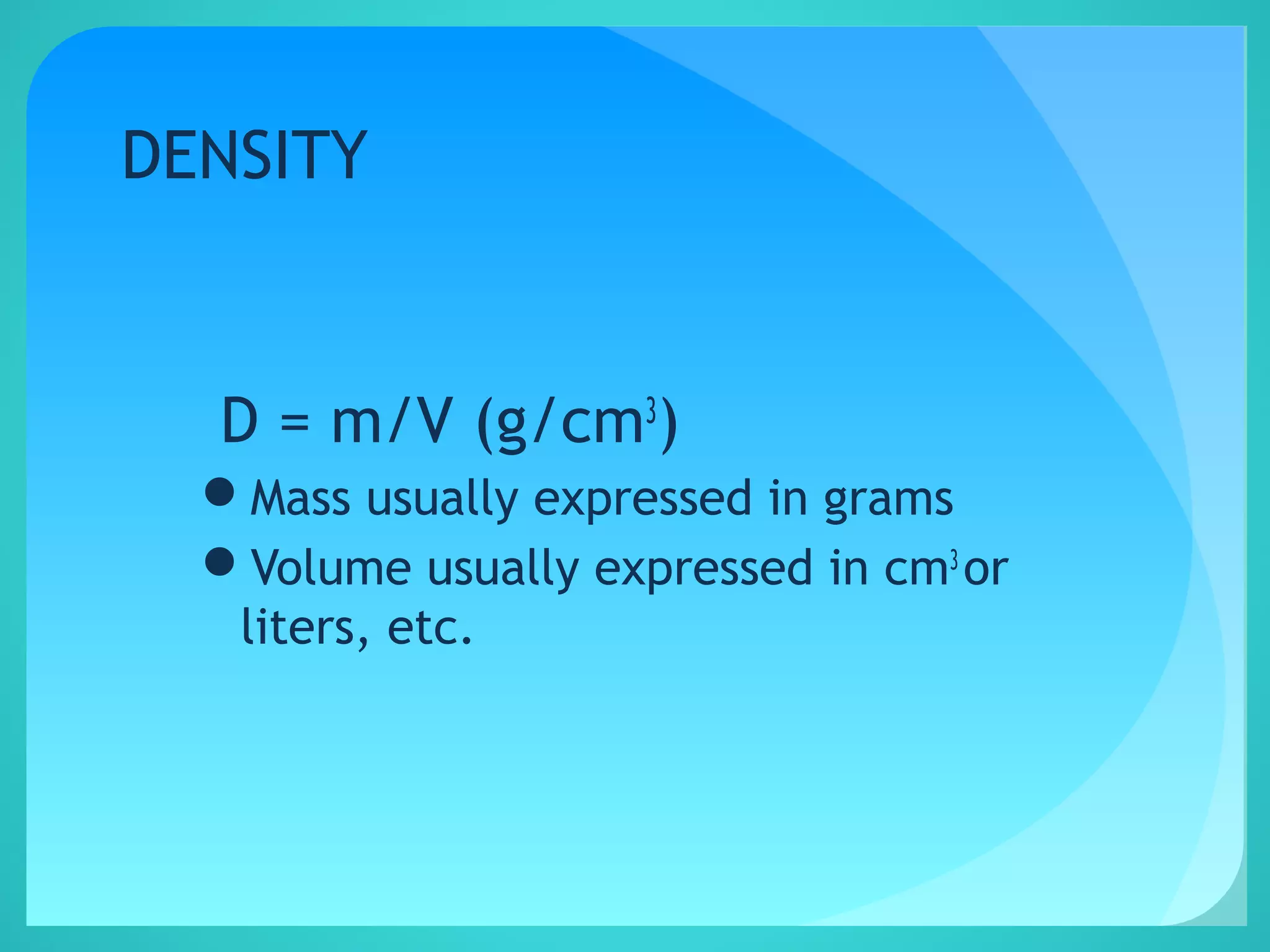
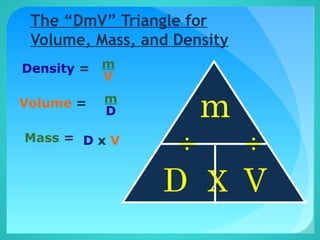
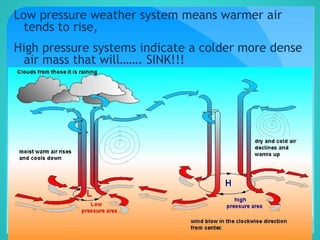
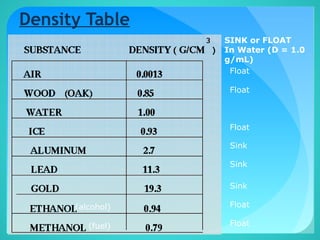
The document discusses the concept of density, defined as mass per unit volume, and its implications for various substances, including solids, liquids, and gases. It highlights factors affecting density such as temperature, pressure, and concentration of dissolved solids. Additionally, it covers the concept of relative density and buoyancy, using examples from nature to explain how density affects behavior in different environments.