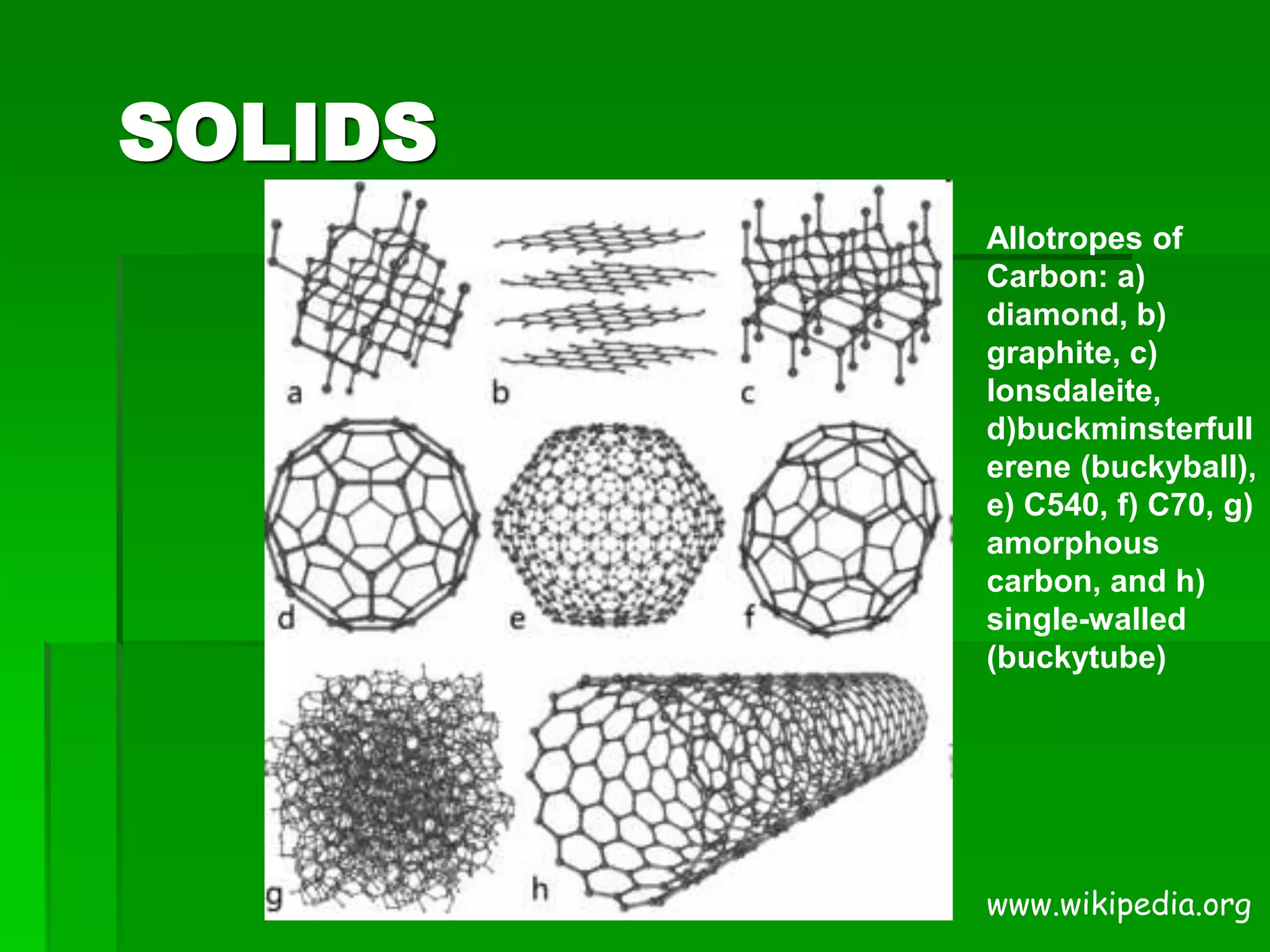
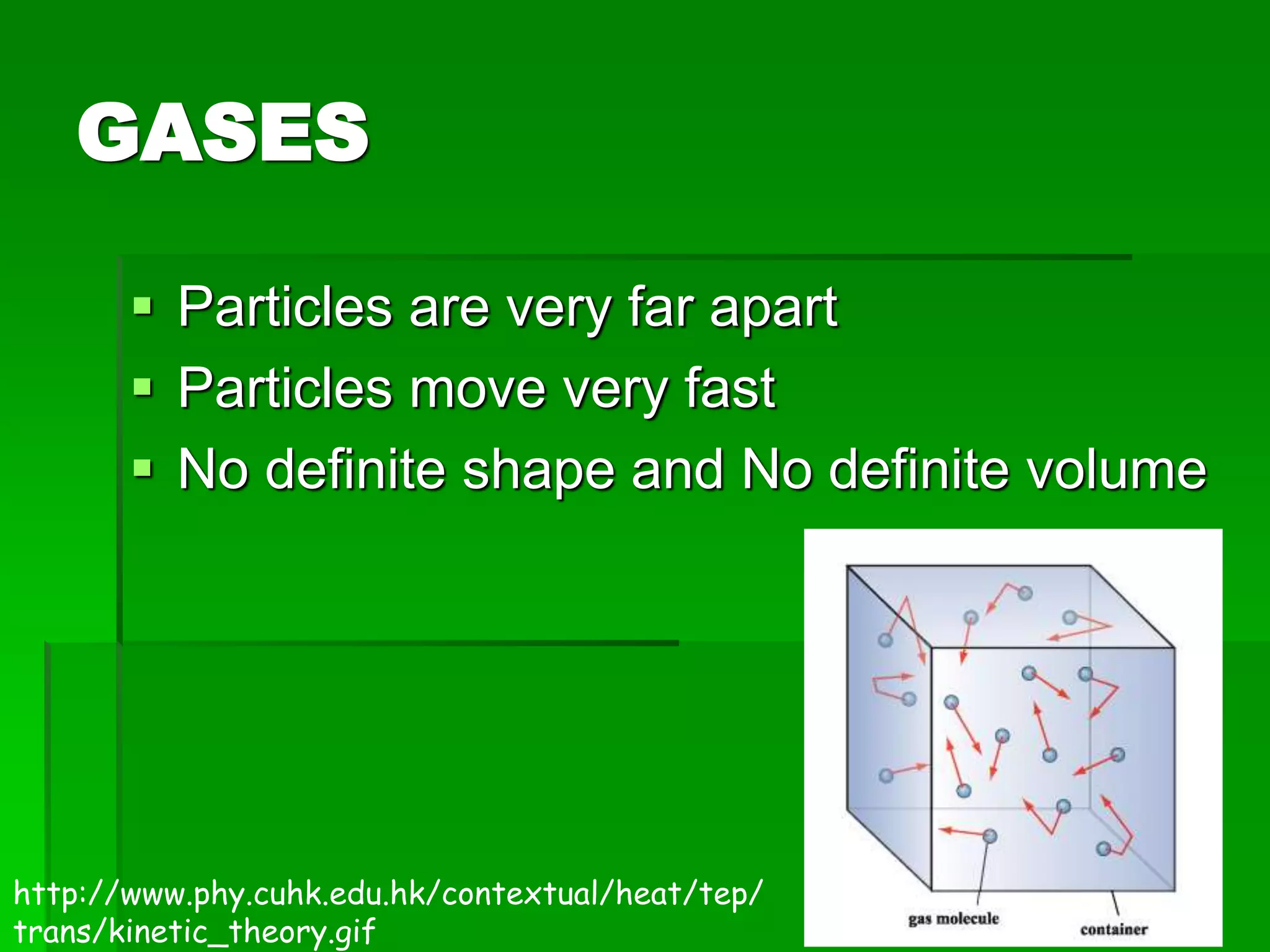
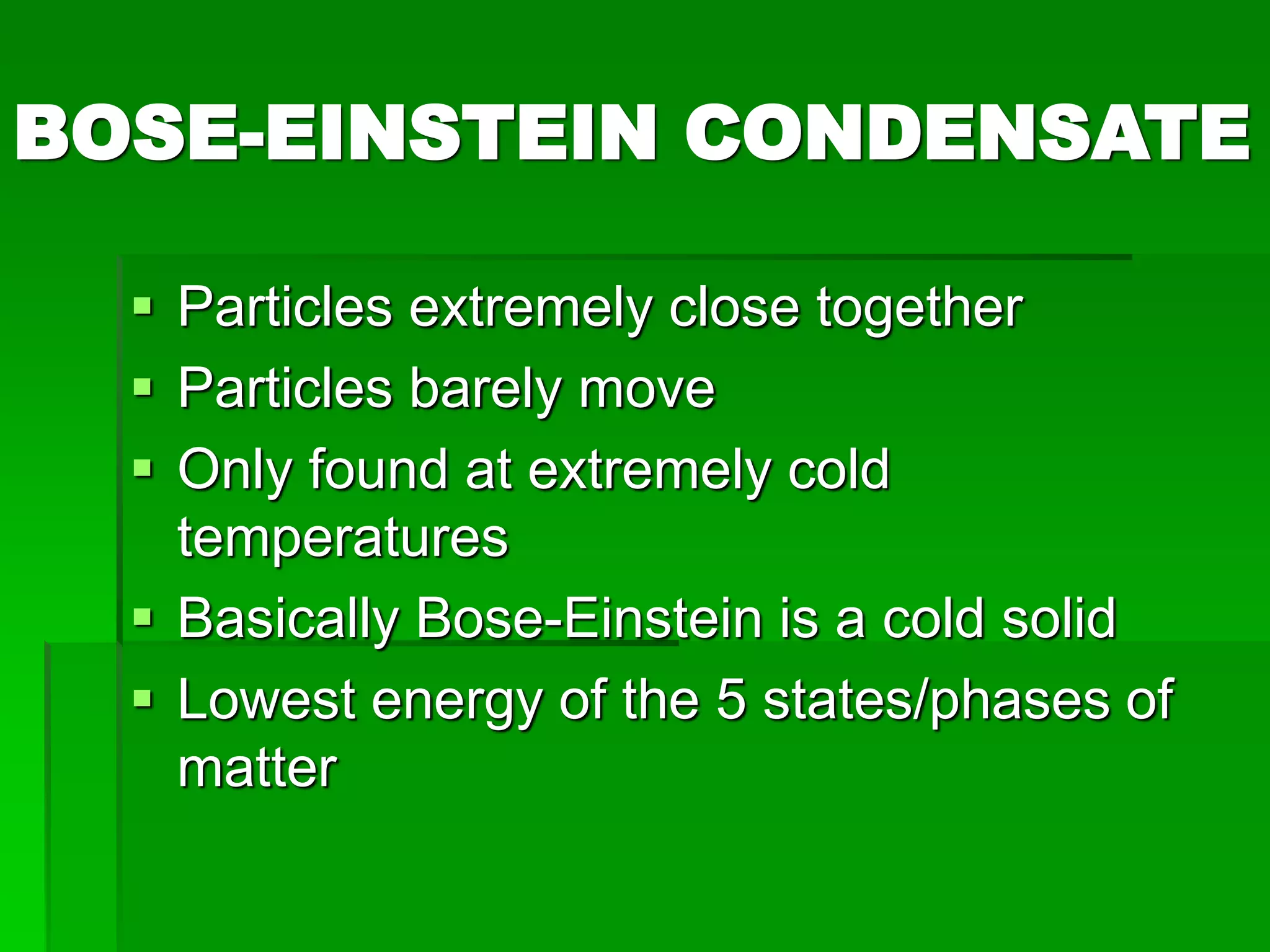
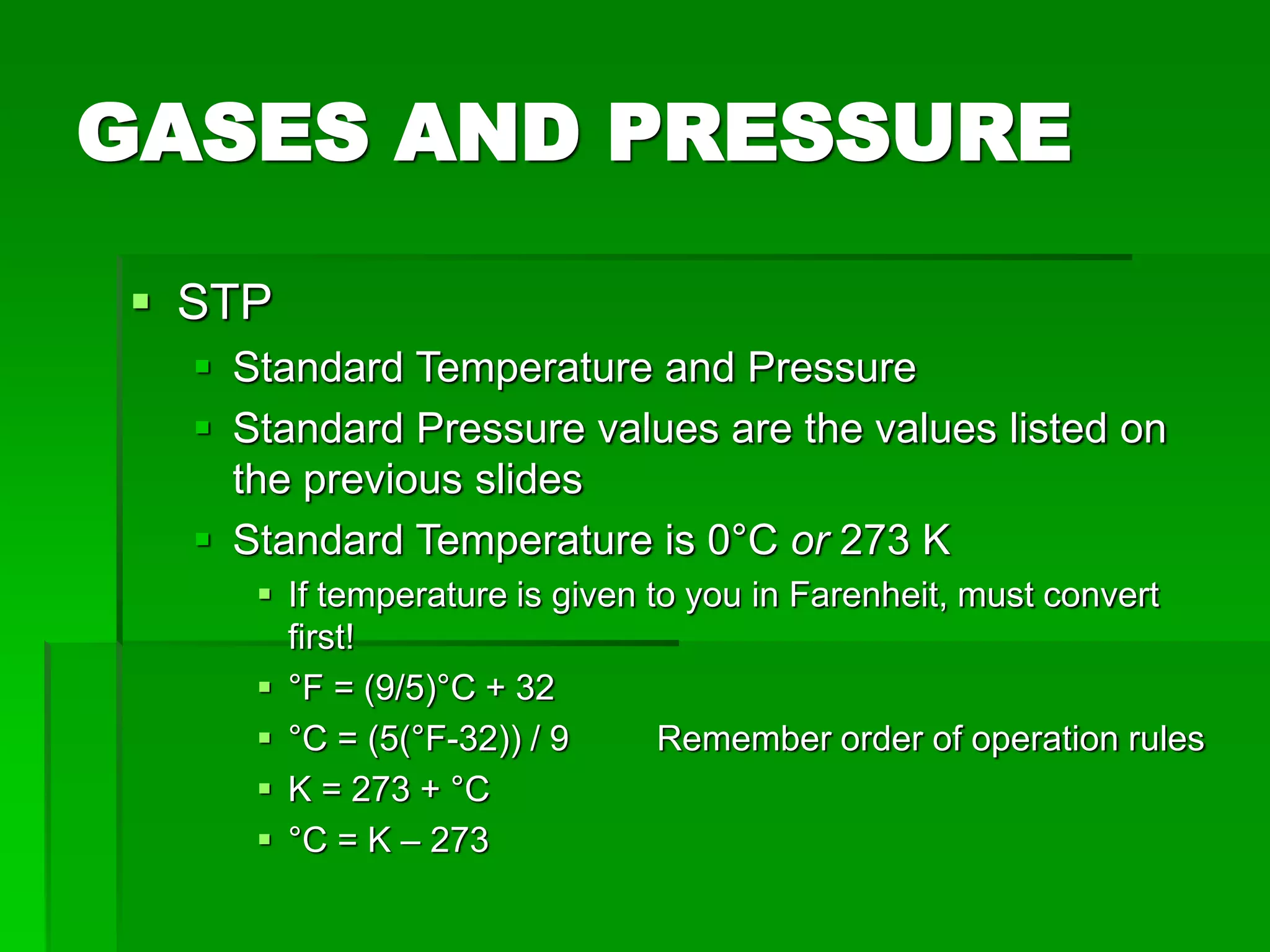
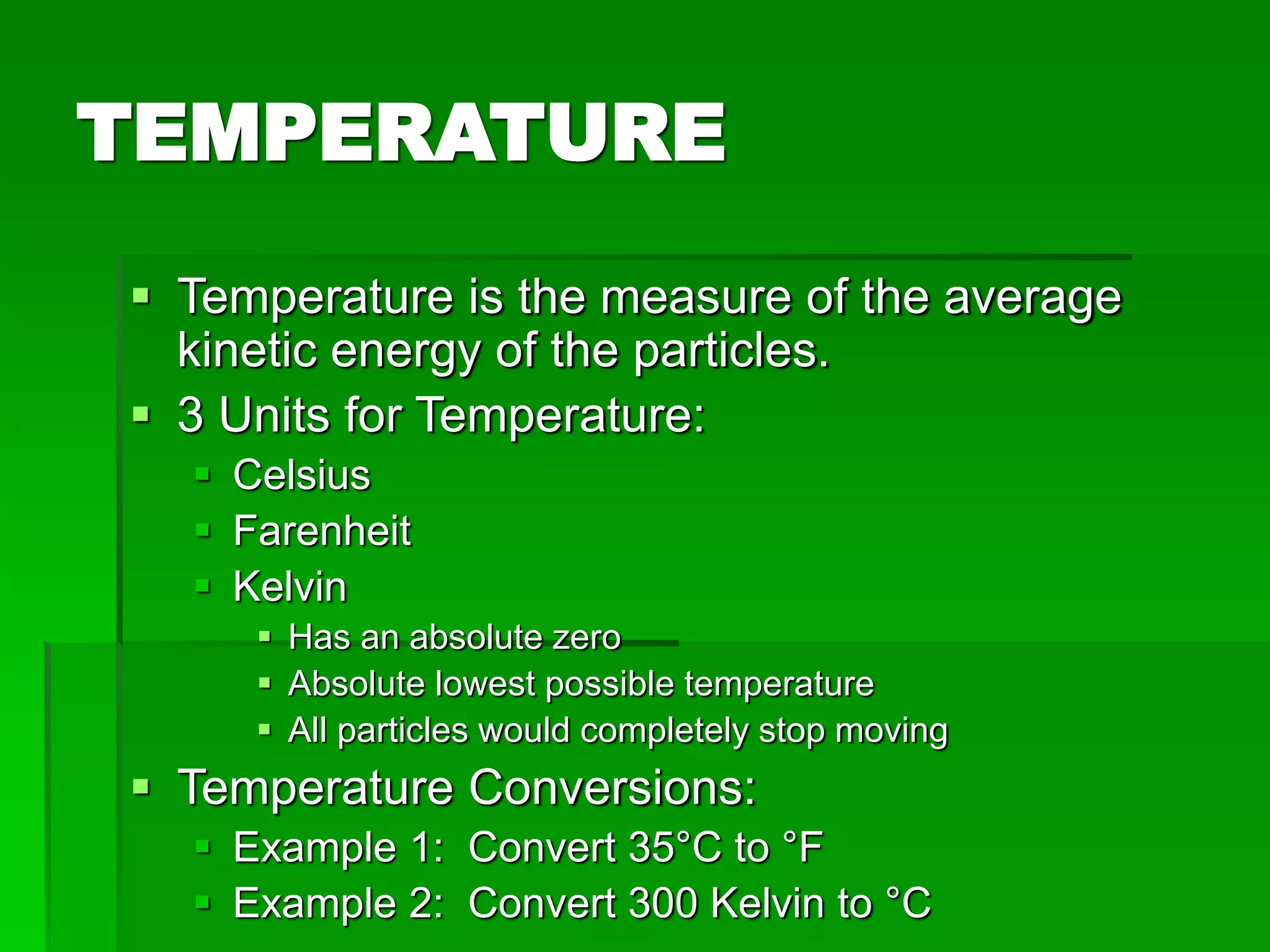
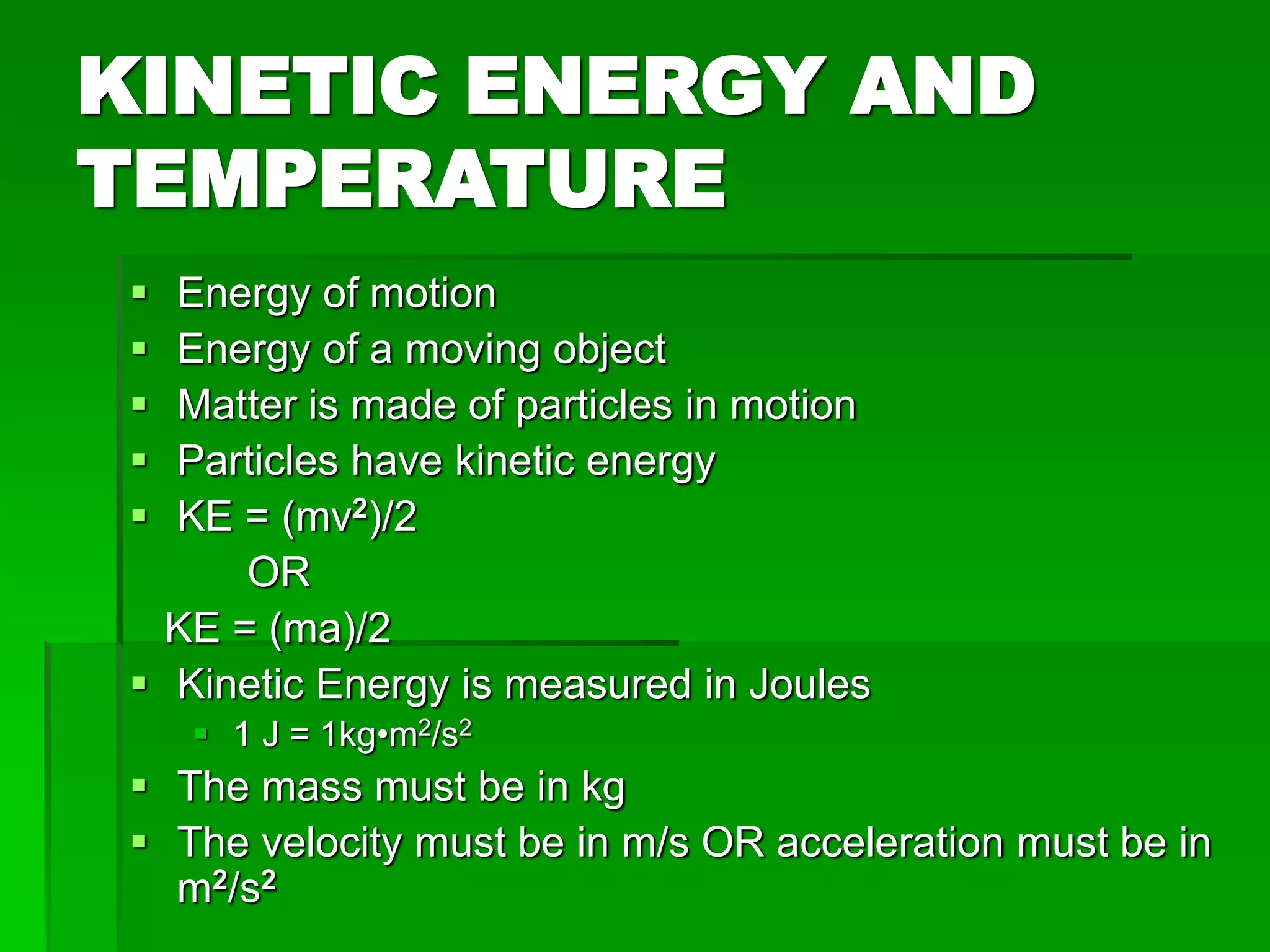
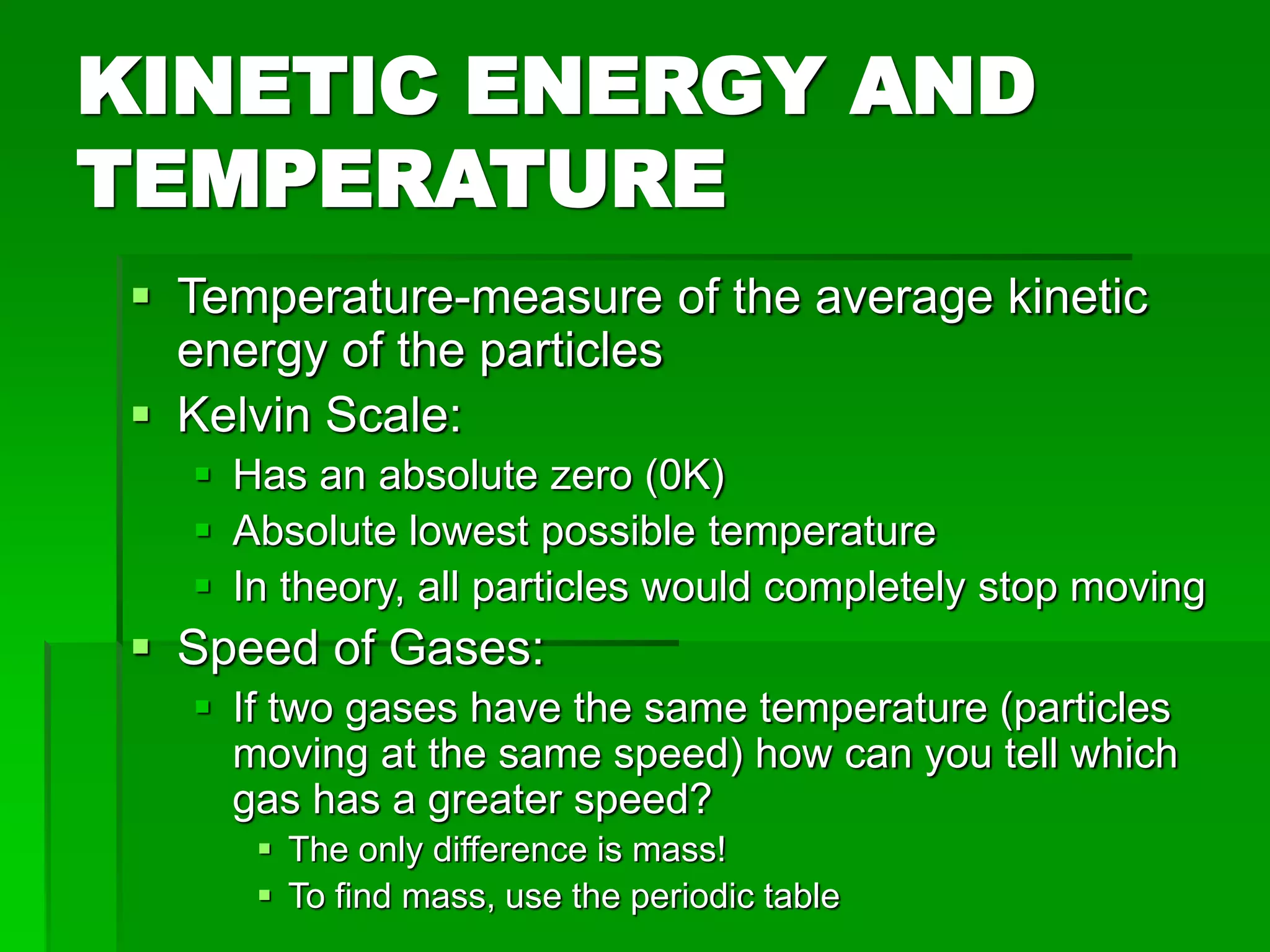
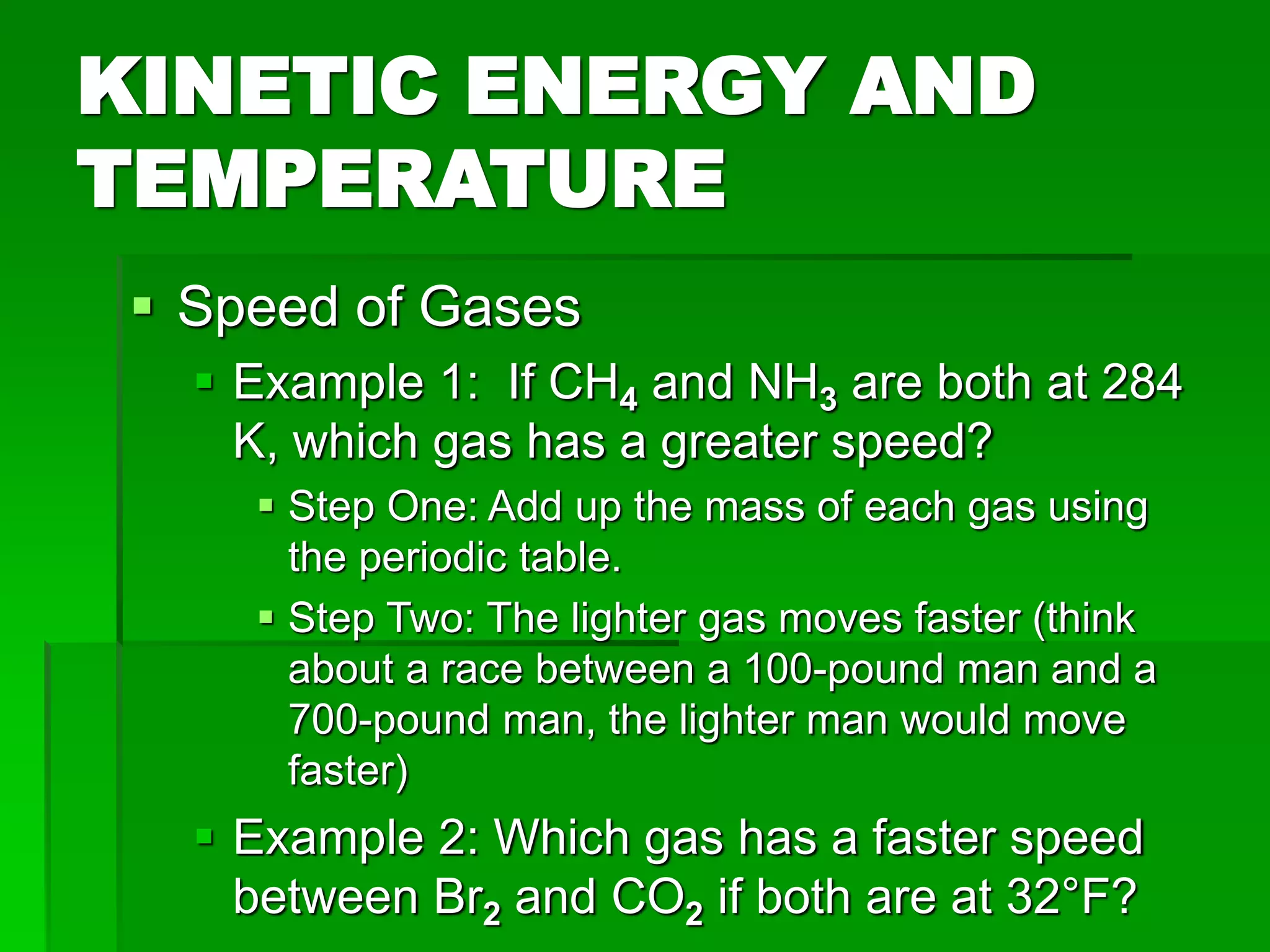
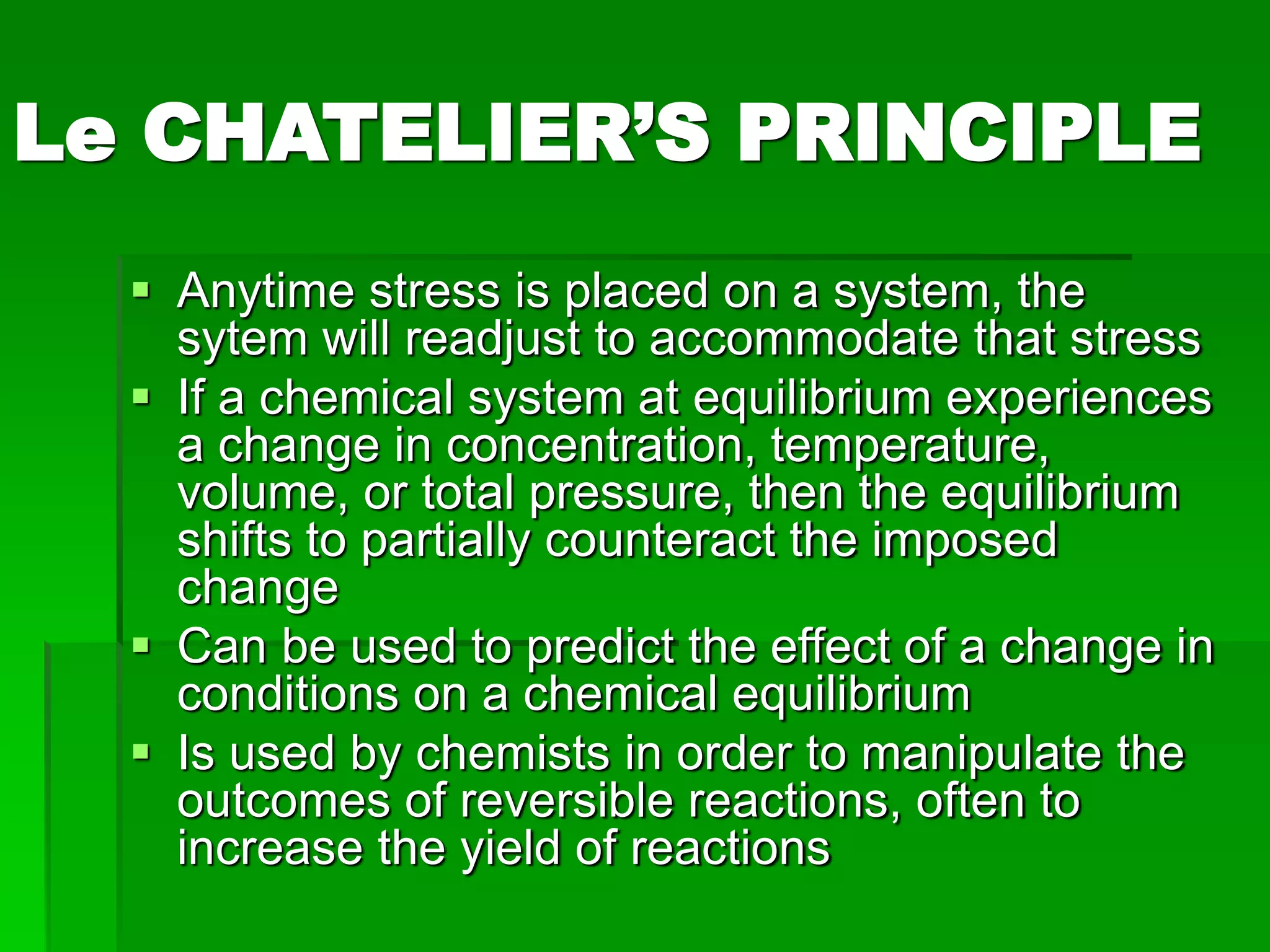
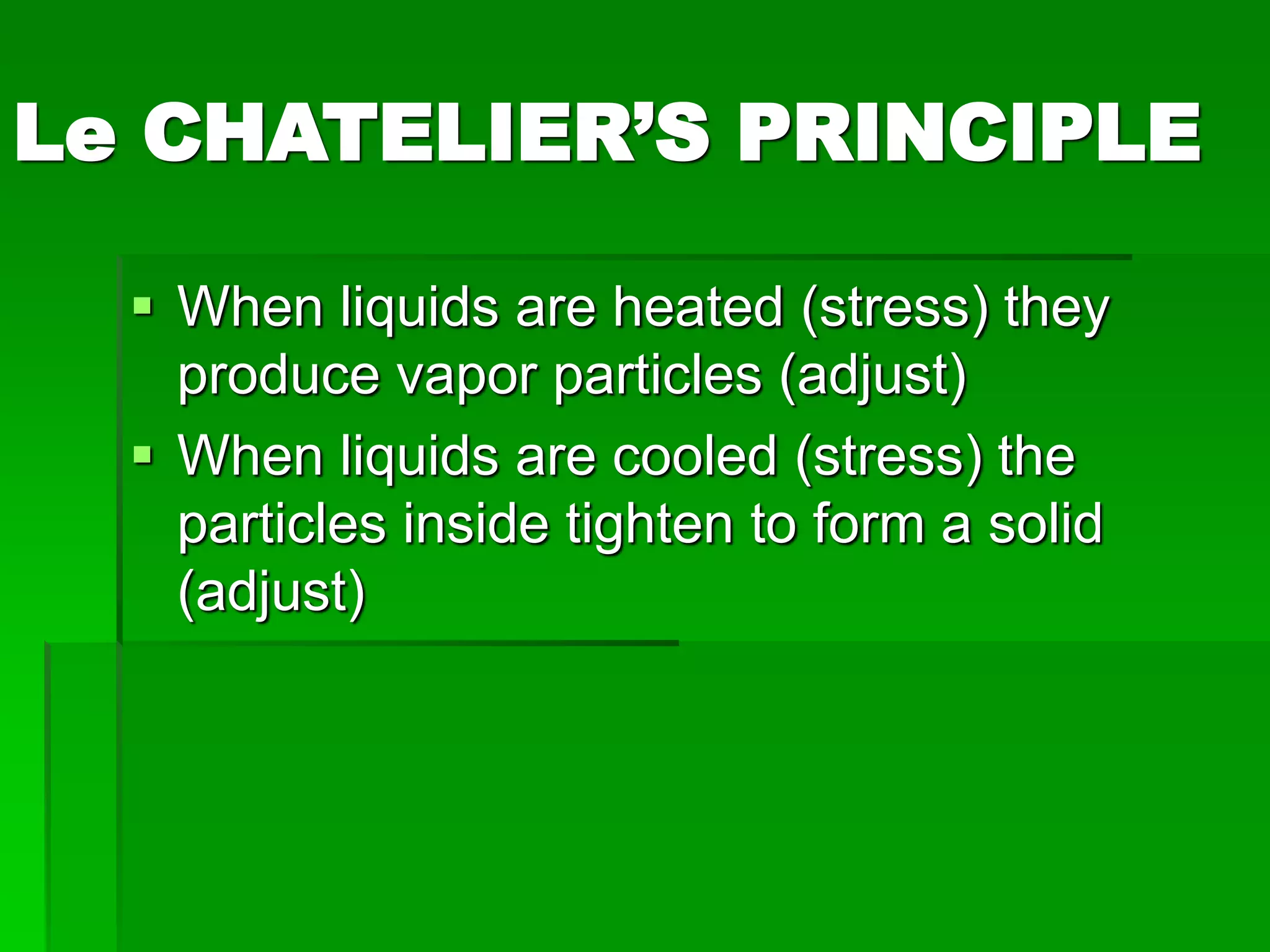
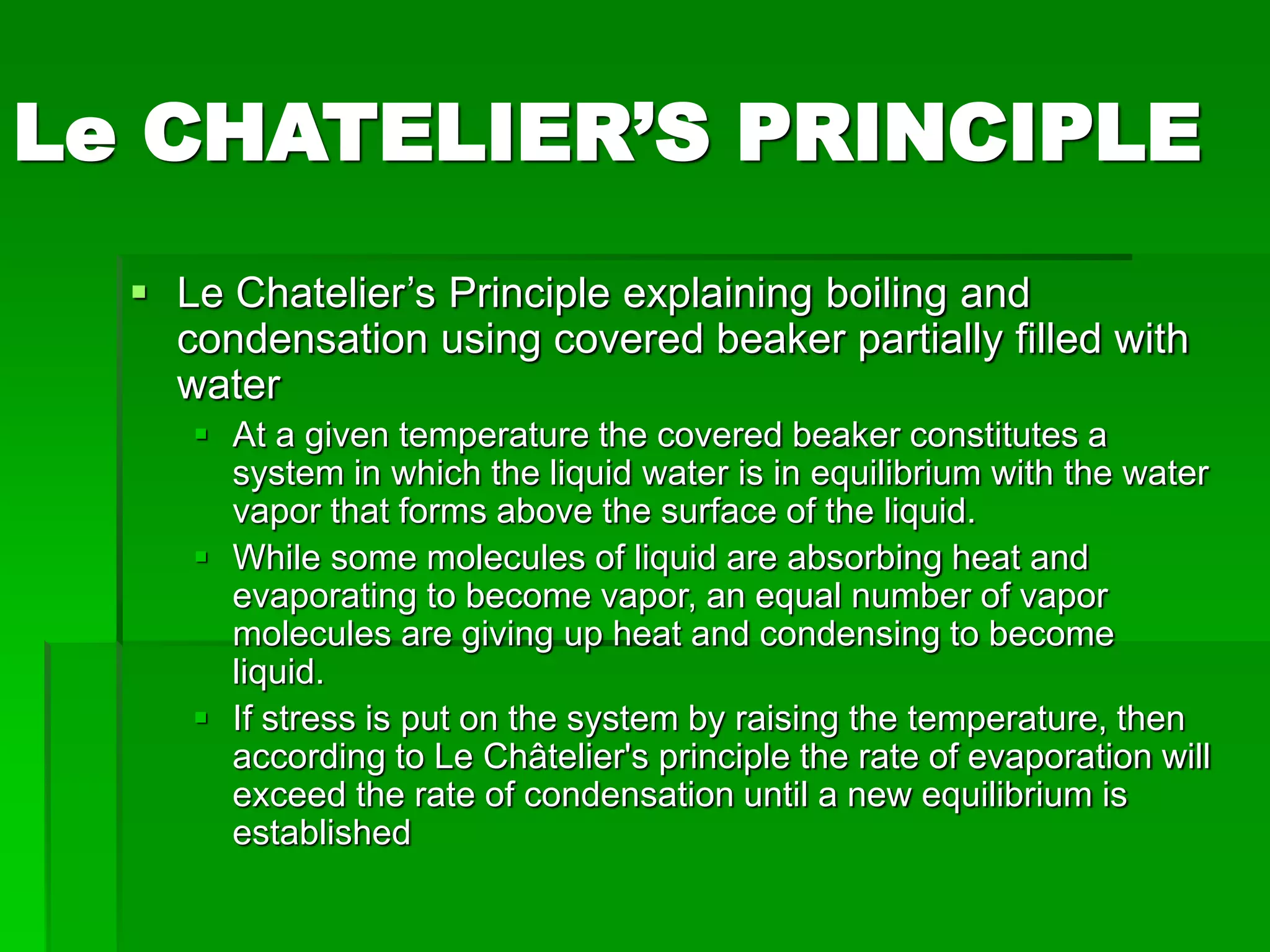
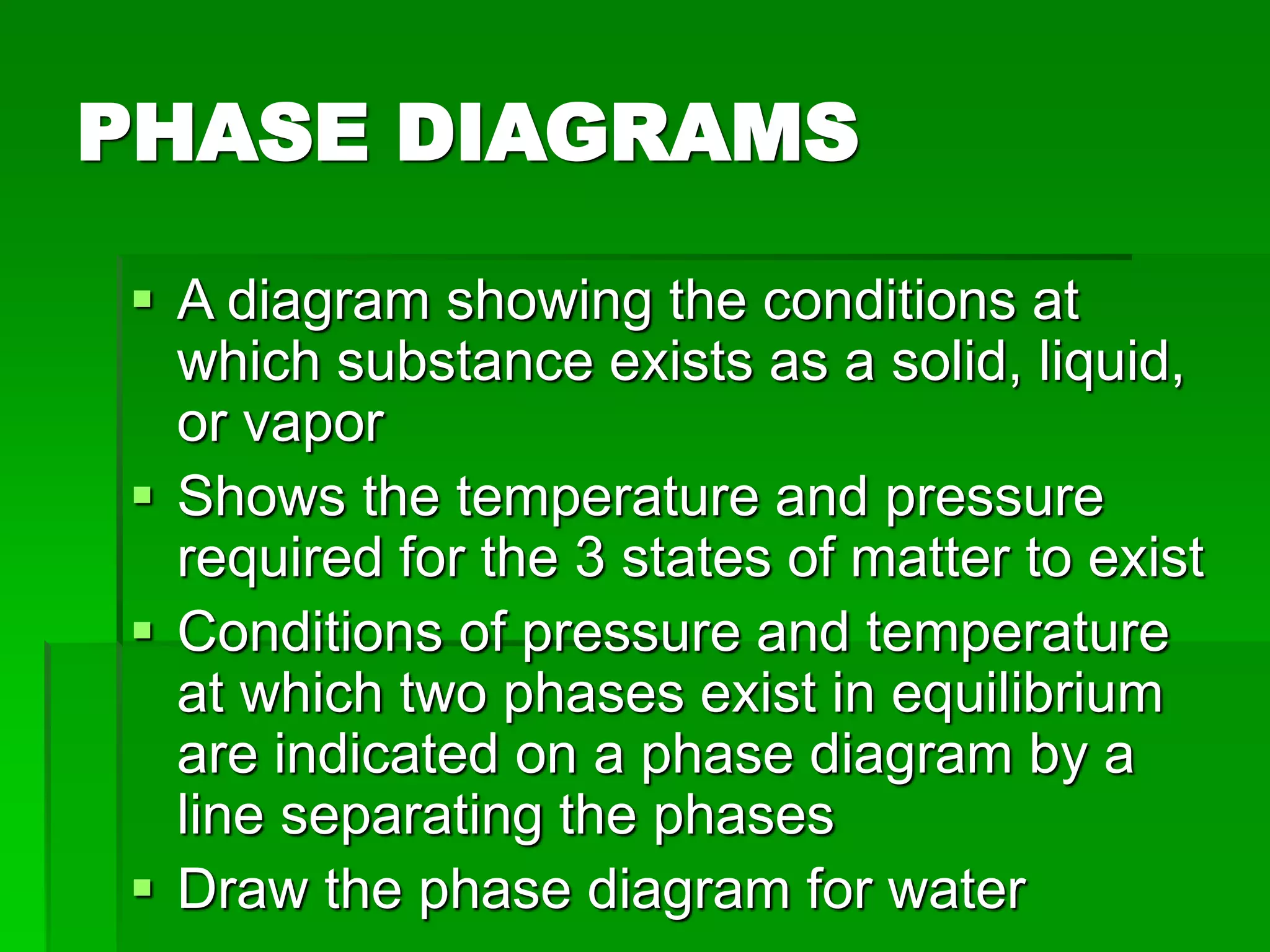
The document provides an overview of kinetic theory and the states of matter. It discusses the following key points:
- Kinetic theory states that all matter is made of tiny particles in constant motion. The behavior of solids, liquids, and gases can be understood through this model.
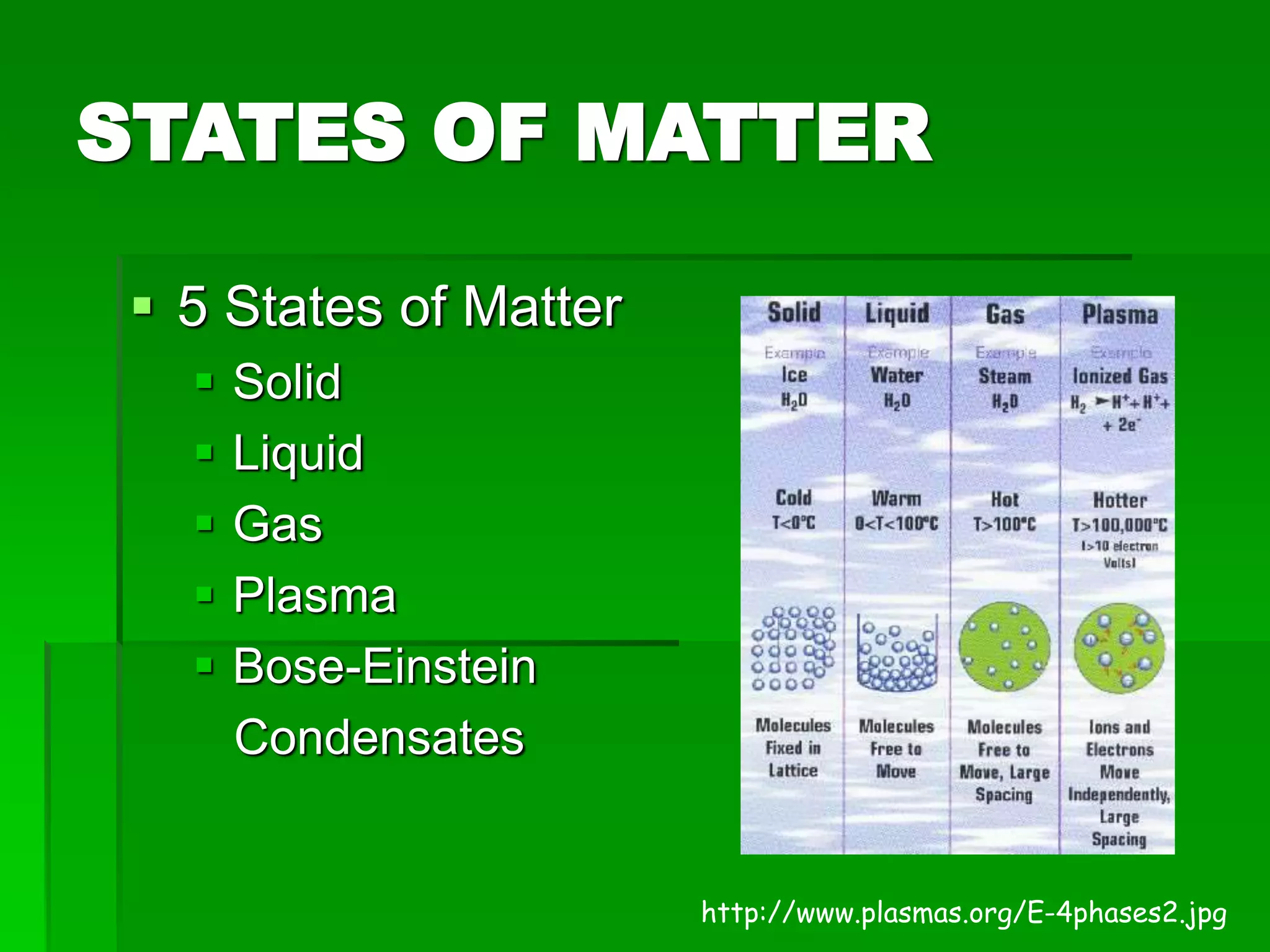
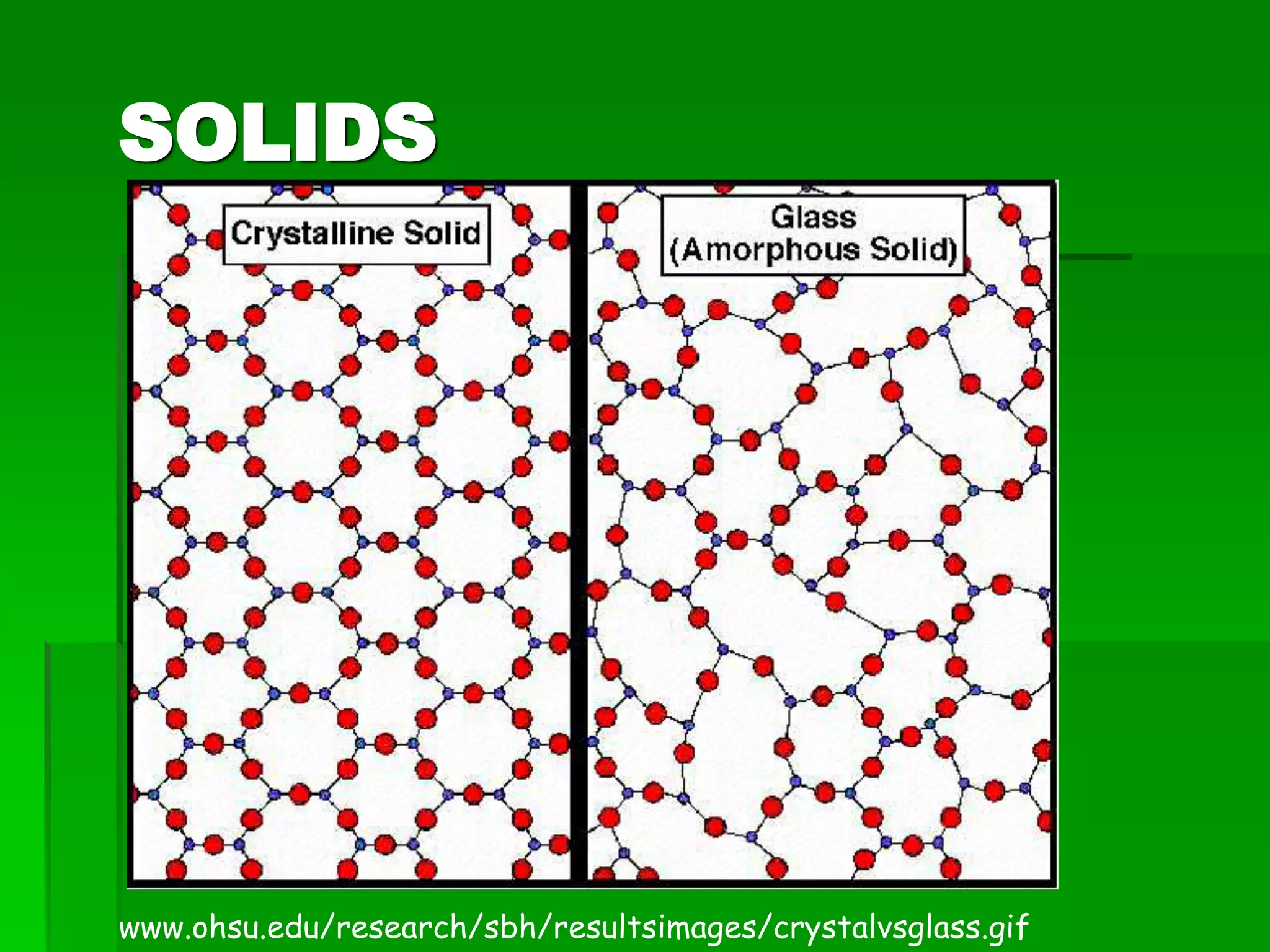
- There are five states of matter: solid, liquid, gas, plasma, and Bose-Einstein condensate. Solids have tightly packed particles that don't move much. Liquids have spread particles that move slowly. Gases have very far apart particles that move very fast.
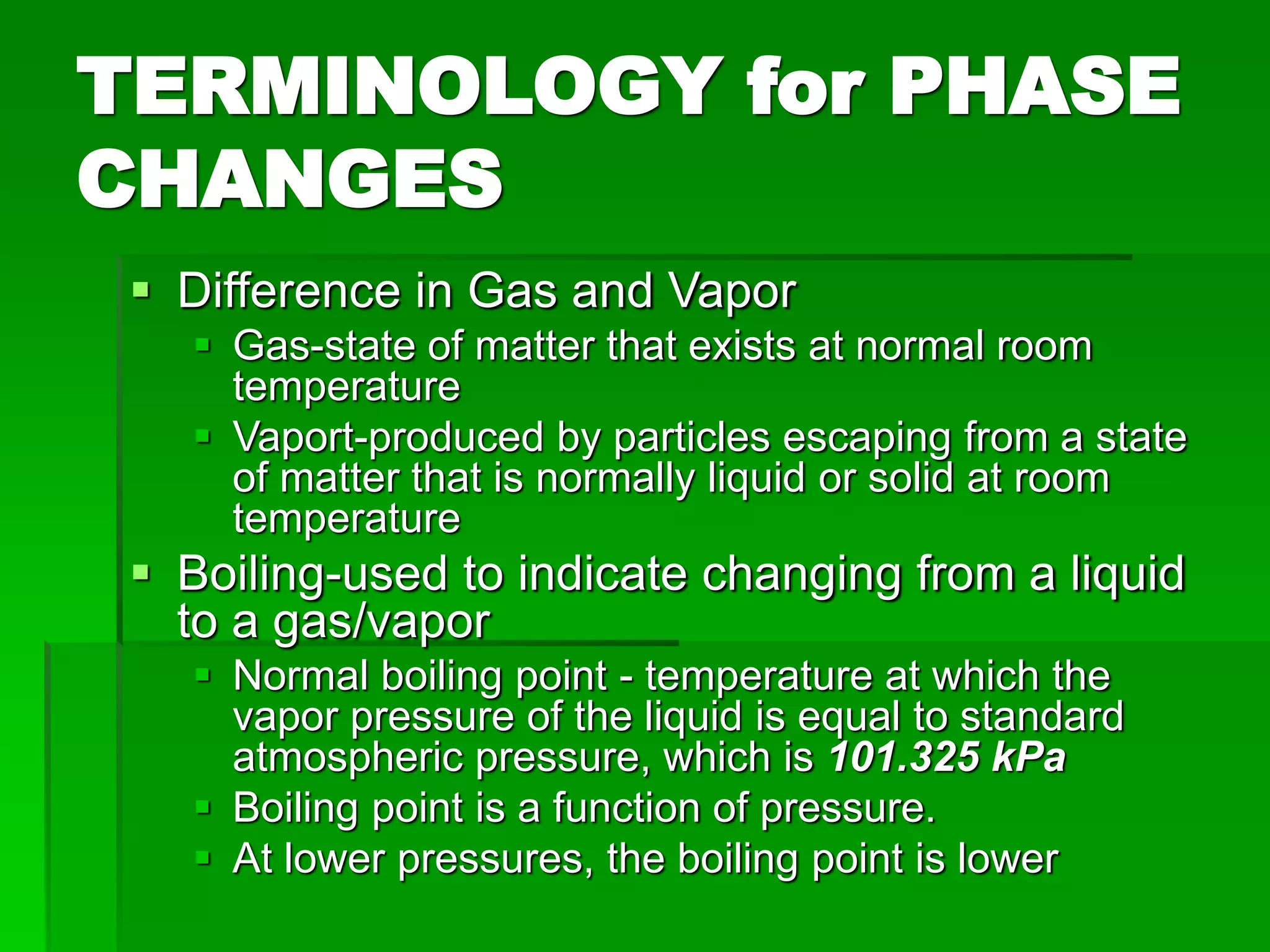
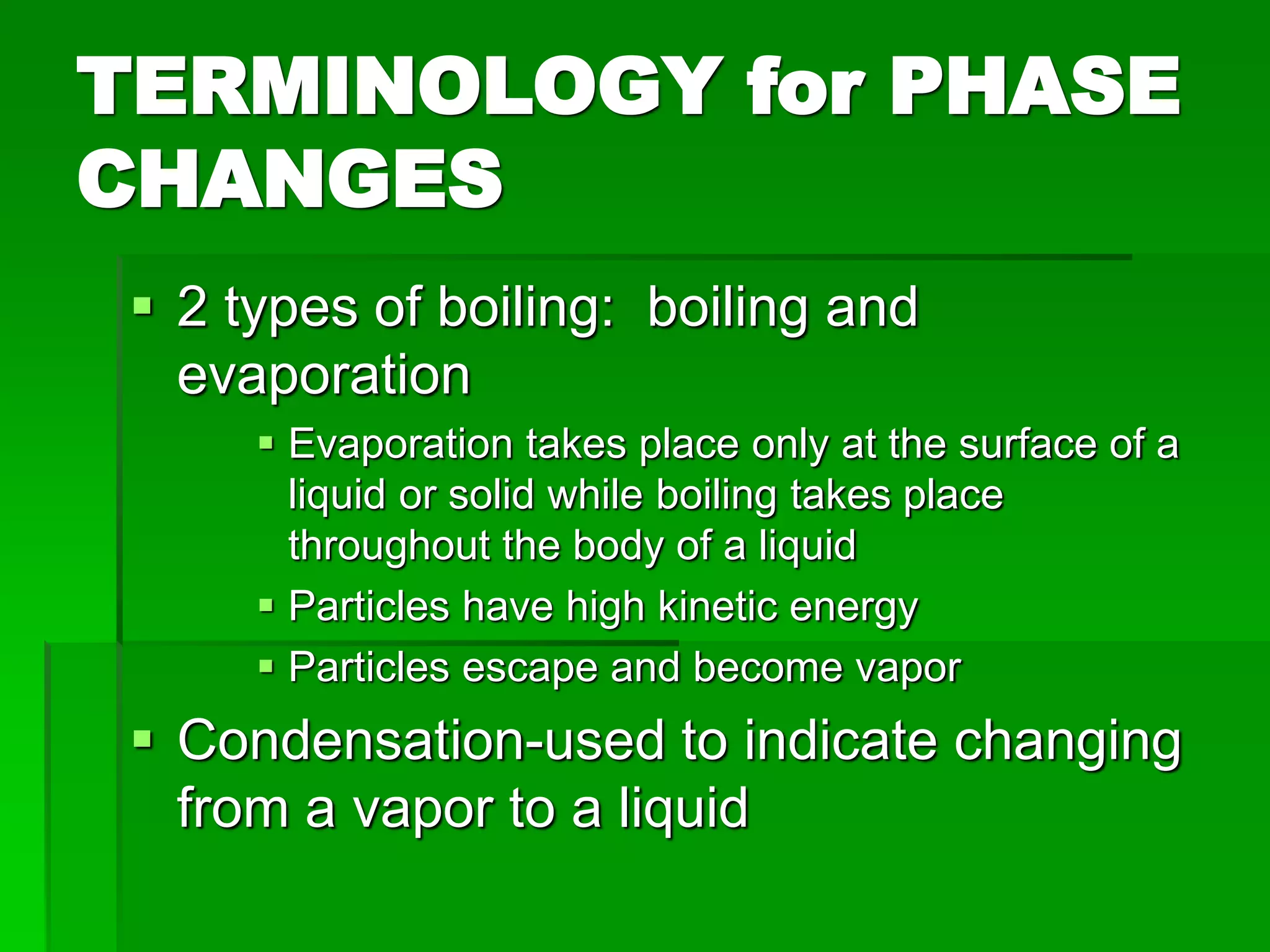
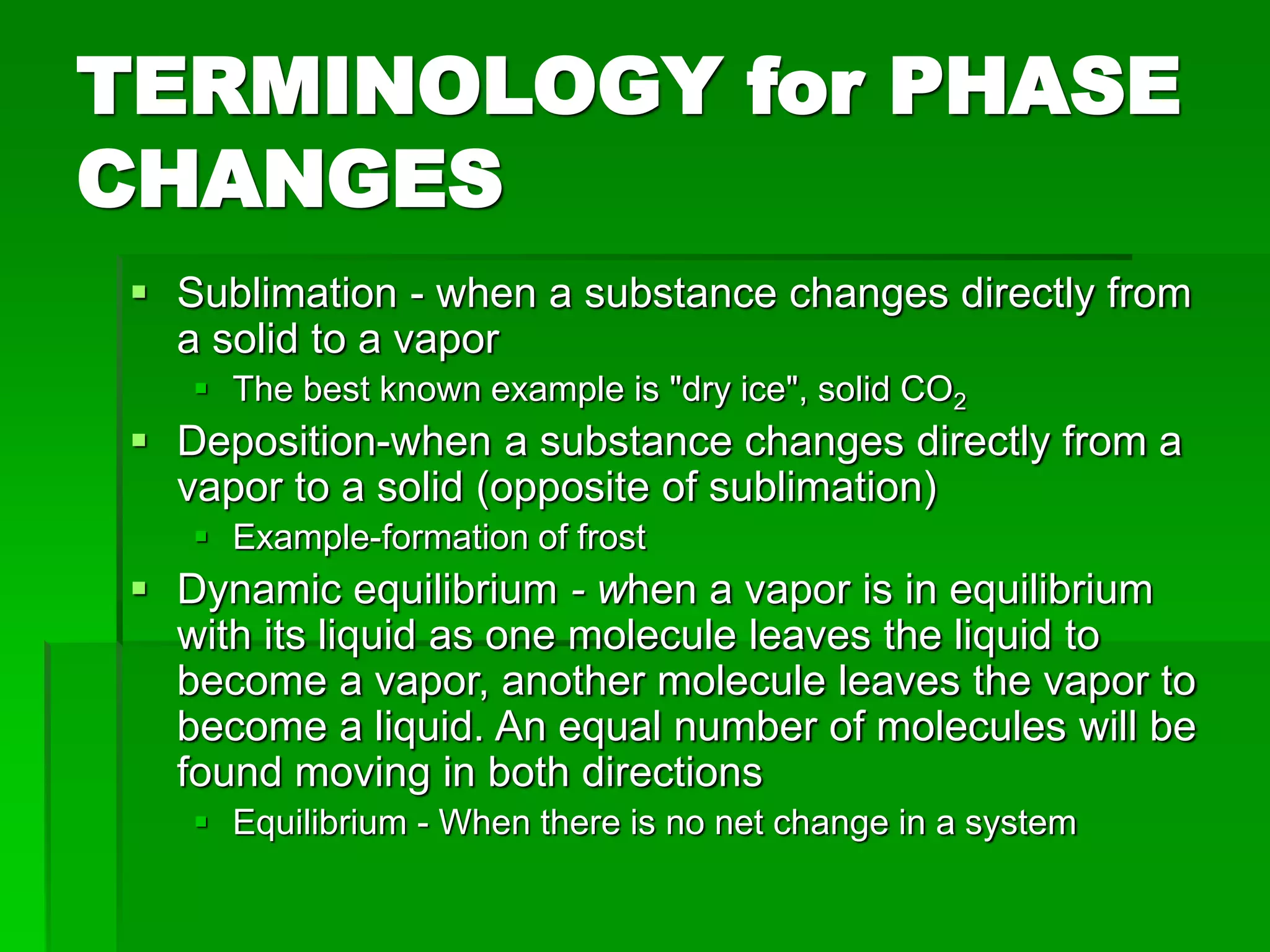
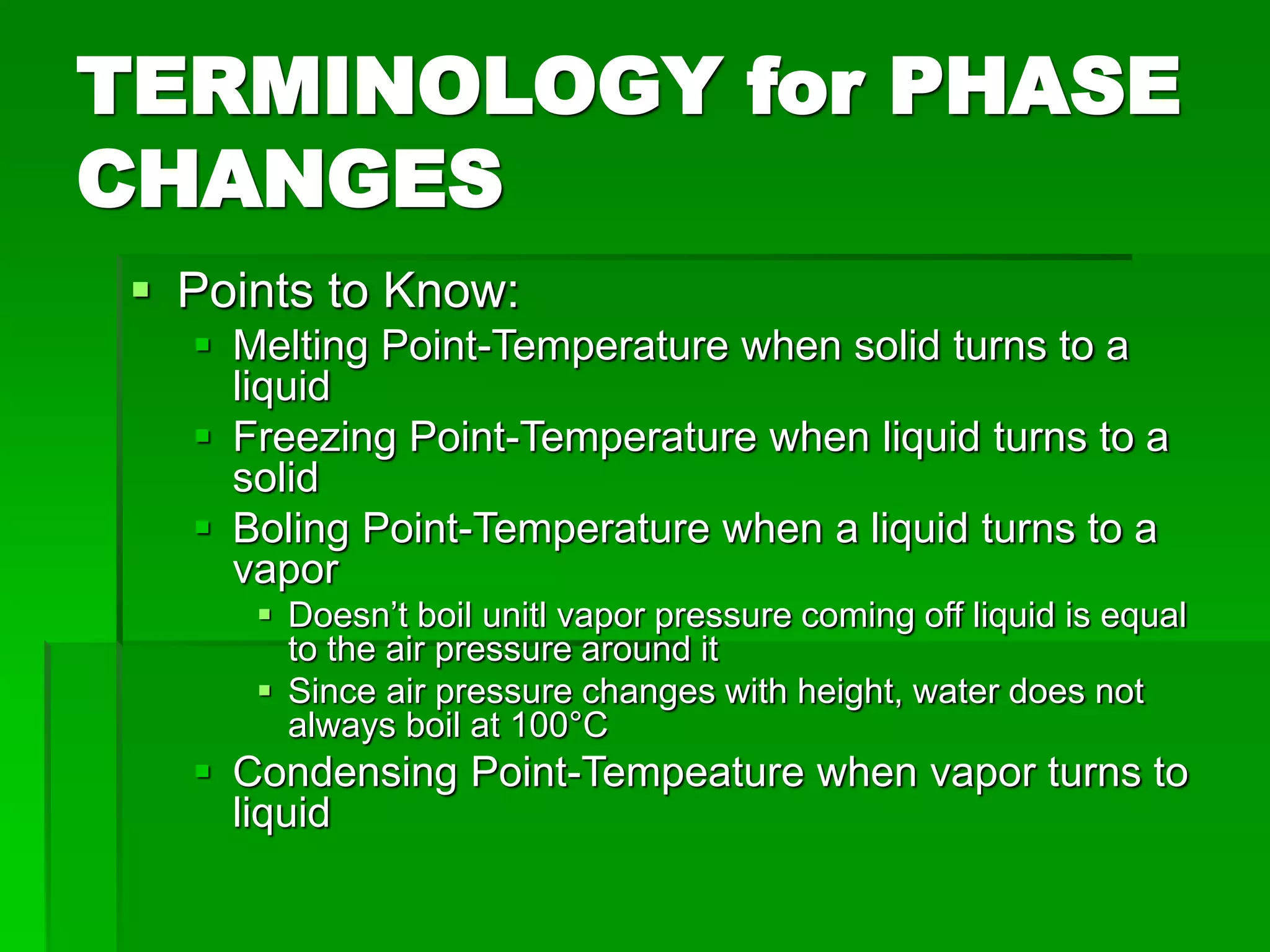
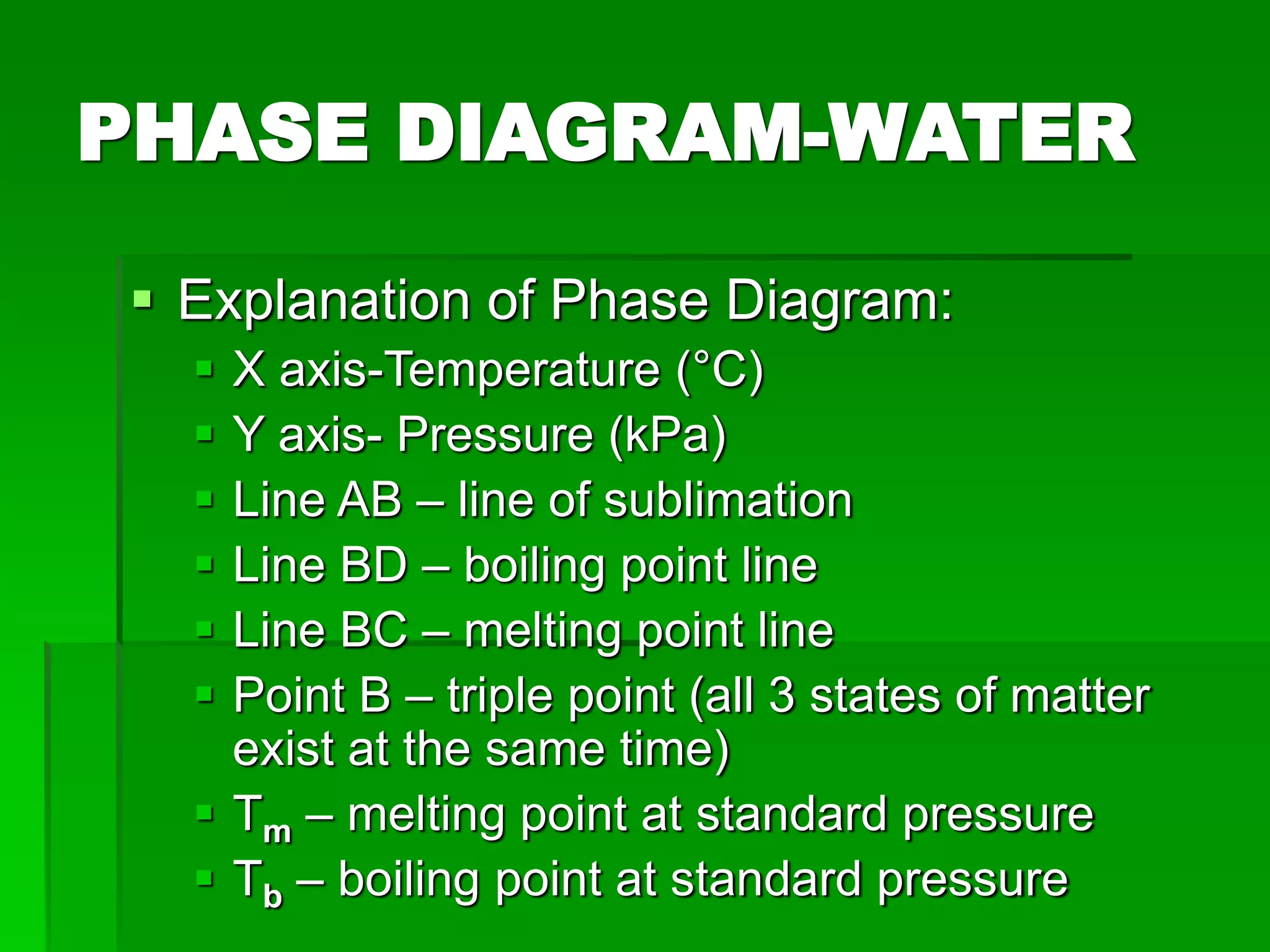
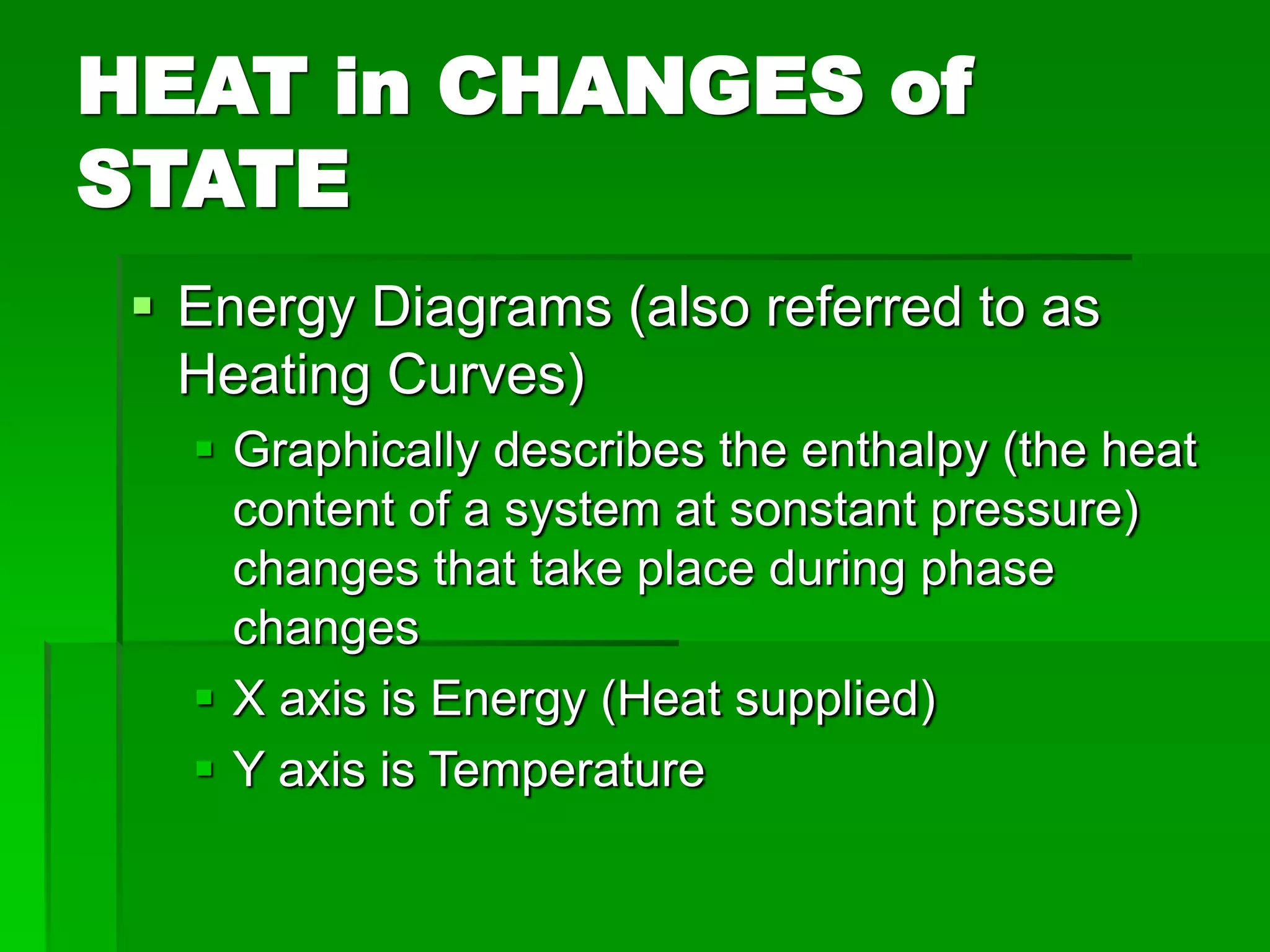
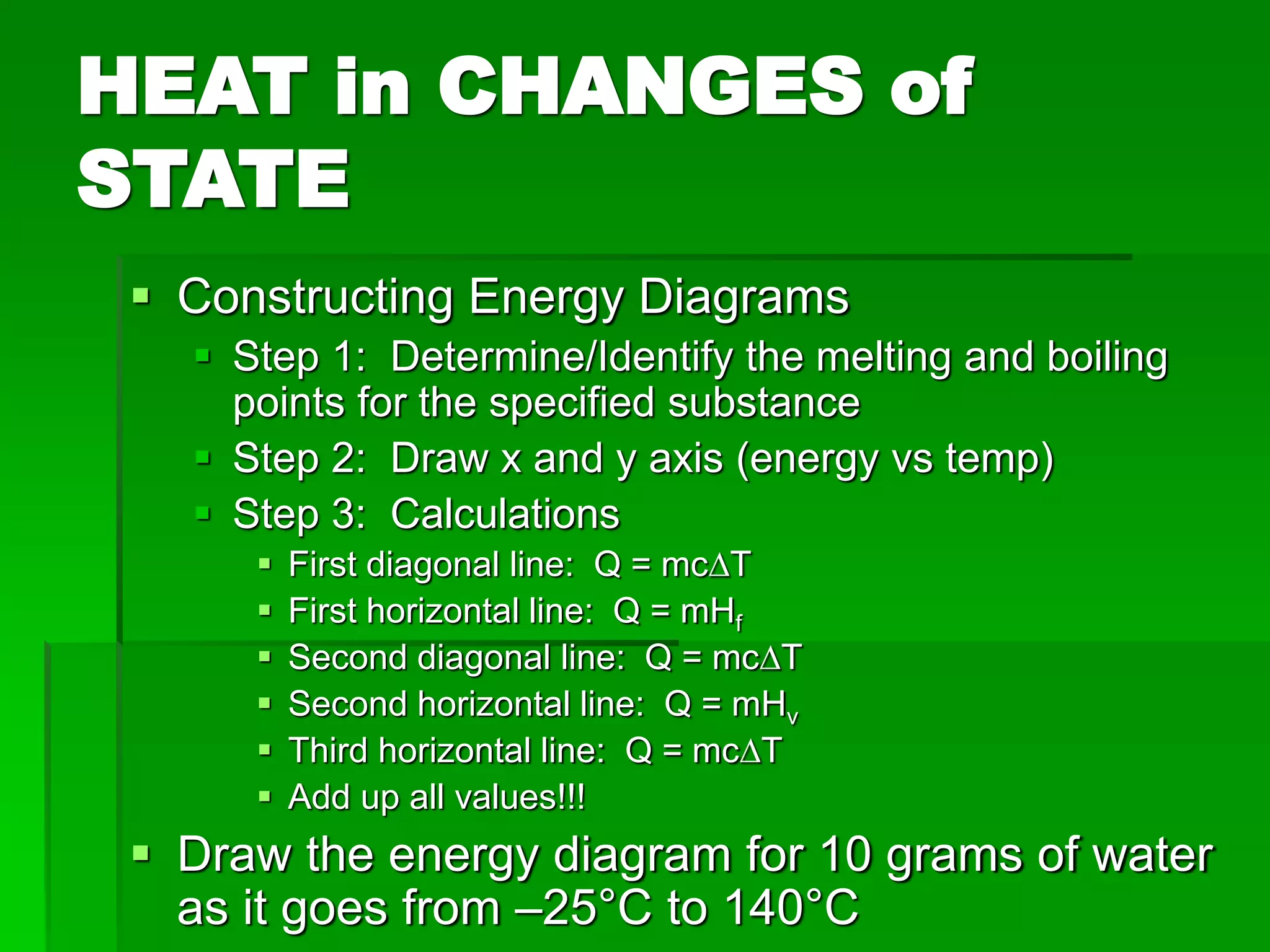
- Phase changes between states, such as melting, boiling, and condensation, occur when sufficient thermal energy is added to or