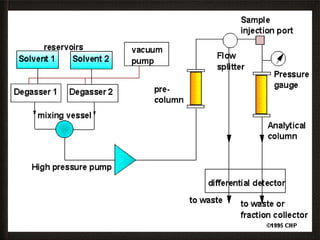
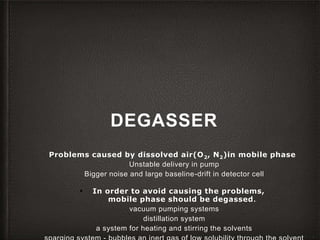
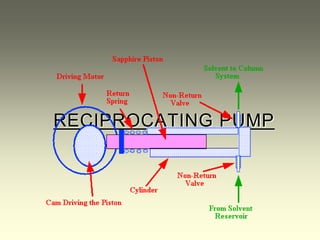
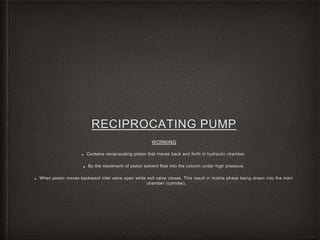
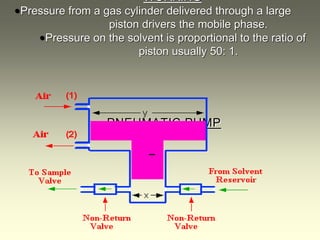
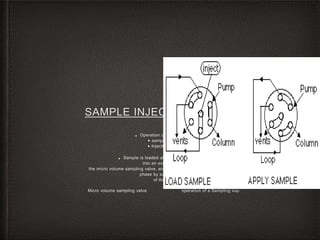
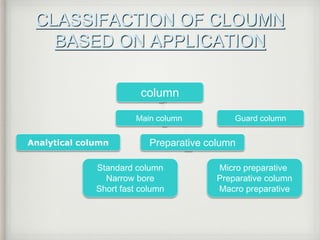
This document provides an overview of high-performance liquid chromatography (HPLC) instrumentation, including its historical development, components, and operation. Key topics include separation mechanisms, types of pumps (reciprocating, displacement, pneumatic), sample injection systems, column classifications, and various detector types. The document emphasizes the significance of each component in achieving effective chromatographic separation and analysis.