Hien nguyen science, vol 315, 493, 2007
- 1. Background
- 2. Background Transpotation depends primarily on petroleum fuel base with a total world usage of refined petroleum products of 90.87 million barrels a day
- 3. A Stable Hydroxide-Conducting Polymer 2015.07.05 히엔 3 J. Am. Chem. Soc. 2012, 134, 10753−10756 Midterm presentation
- 4. Pt cost efficiency Loss Pt surface Major problems of ORR - Platinum (Pt) or Pt-based catalysts often contribute 20-25% to the cost of PEMFC systems. - Efficiencies of present PEMFC systems range between 35-45%, although the theoretical limits are nearly 80% efficiency for low temperature fuel cells like PEMFC. - The dissolution and/or loss of Pt surface area in the cathode. Introduction OH– bonds tightly to Pt surface atoms, leaving less room for O2 to adsorb onto Pt active sites. Blocking active sites results in hinders the rate of cathodic reaction.
- 5. Approach This research examined selected cathode materials with well- characterized surfaces so that the mechanism of action can be attributed to a specific property (at the atomic and molecular level) of the surface. In this way, we can determine: (i) Whether the kinetics of the ORR are structure-sensitive (ii) The composition of the topmost surface atomic layers (the segregation profile) (iii) How alloying [usually described in terms of the ligand effect or/and ensemble effect] alters the chemical properties of the surfaces.
- 6. Result & Discussion Surface characterization of the Pt3Ni single crystals (LEEDS) low-energy electron diffraction spectroscopy. (D to F) (LEIS) low-energy ion scattering (B) Composition of the outemost layer: pure 100% Pt (Pt skin structures) (UPS) synchrotron-based high- resolution ultraviolet (C) ≠ surface electronic structure (CV) Cyclic voltammetry as compared to the voltammetry of the corresponding Pt single crystal (gray curves). RHE reversible hydrogen electrode. Fig1. A combination of in situ and ex situ surface-sensitive probes and density functional theory (DFT) calculations was used to study ORR on Pt3Ni(hkl) single- crystal surfaces. 1. Which surface properties govern the variations in reactivity of PtNi catalysts? 2. How surface structures, surface segregation, and intermetallic bonding affect the ORR kinetics? Answer
- 7. Result & Discussion LEEDS surface characterization of the Pt3Ni(111). The green dots in this LEEDS pattern (left) for a single crystal of Pt3Ni(111) reveal a tightly packed arrangement of surface atoms that repels platinum-grabbing hydroxide ions and boosts catalytic performance. Selling point of Fig 1
- 8. Result & Discussion 8 (2A,A’) Surface x-ray scattering (SXS) was used to characterize the position and near-surface composition of the alloy in situ 1st layer 100% Pt; 2nd layer 52% Ni; 3th layer 87% Pt. At 0.05V the potential was cycled both the Pt3Ni(111) surface structure and the segregation profile are completely stable. (2B) Surface coverage calculated from cyclic voltammograms of Pt3Ni(111) and Pt(111). with the onset of adsorption on Pt(111)-skin, which consists of the same surface density of Pt atoms as Pt(111), a dramatic negative shift (≈0.15 V) in Hupd formation and positive shift (≈0.1 V) in OHad formation occurred relative to Pt(111). on Pt3Ni(111), the fractional coverages by Hupd and Ohad were dramatically reduced by 50% relative to Pt(111), which is in agreement with the large downshift (0.34 eV) of the d-band center position on the Pt-skin structure (1C) (2C) Position of the d-band centers to the fractional coverages of adsorbed hydrogen (H+ + e– = ӨHupd, where Hupd refers to the underpotentially deposited hydrogen) between 0.05 < E < 0.4 V, where E is the applied potential, Hydroxyl species (2H2O = OHad + H3O+ + e–, where OHad is the adsorbed hydroxyl layer) above 0.6 V
- 9. The DFT calculations show a positive shift of ∆U° = 0.10 V when the sublayer has 50% Ni atoms. The experiment and theory thus reach an excellent and quantitative agreement in this case and clearly establish an electronic effect of subsurface Ni on the Pt-OH chemical bonding. The kinetics of O2 reduction are determined by the number of free Pt sites available for the adsorption of O2 (1 – Өad) and by the ∆Gad of O2 and reaction intermediates on metal surfaces precovered by OHad. the change in the reversible potential the rate of the ORR:
- 10. (E) ORR currents measured on Pt3Ni(111), Pt(111), polycrystalline Pt surfaces. The positive potential shift of 100 mV in electrode half-potential (∆E1/2) between ORR polarization curves measured on Pt poly and Pt3Ni(111) surfaces. ӨOHad ↓ on the Pt-skin structure, the key parameter that determines the unusually catalytic activity of Pt3Ni(111) is the low coverage by OHad [i.e., the (1 – Өad) equation 2]. (D) Green scale refers to hydrogen peroxide production in designated potential region Because of the lower coverage by Hupd, the production of peroxide is substantially attenuated on the Pt-skin surface. on Pt3Ni(111) the fuel cell relevant potentials (E > 0.8 V), the observed catalytic activity for the ORR is the highest that has ever been observed on cathode catalysts, including the Pt3Ni(100) and Pt3Ni(110) surfaces. Result & Discussion
- 11. Fig 3. Influence of the surface morphology and electronic surface properties on the kinetics of ORR. Result & Discussion Pt3Ni(100)-skin < Pt3Ni(110)- skin <<< Pt3Ni(111)-skin activities increasing in the order Pt(100) << Pt(111) < Pt(110) (Fig. 3). Different electronic structure (|Dd[111]| = 0.34 eV, where |Dd[hkl]| is the d-band center shift), the ORR is being enhanced by factor of 10 on Pt(111)-skin relative to that on Pt(111).
- 12. The Pt3Ni(111) surface has an unusual electronic structure (d-band center position) and arrangement of surface atoms in the near-surface region. This causes a weakening of the bonds between the Pt surface atoms and the OH– molecules. The weakening increases the number of active sites available for O2 adsorption. The overall effect generates an increase in specific activity for cathodic reaction: 10 times more active than the Pt(111) surface . 90 times more active than state-of-the-art Pt/C catalysts currently used in fuel cells. The next step is to engineer nanoparticle catalysts with electronic and morphological properties that mimic the surfaces of pure single crystals of Pt3Ni(111). the amount of Pt will be reduced without a loss in cell voltage, while also maintaining the maximum power density. Conclusion
- 14. S. Guo, S. Zhang, S. Sun, Angew. Chem. Int. Ed. 2013, 52, 8526-8544.
- 15. Z.-Y. Yang et al. J. Mater Chem. A. 2014, 2, 2623-2627. Bean pod shaped Fe-C-N Graphene based Fe-C-N H. R. Byon et al. Chem. Mater. 2011, 23, 3421-3428. Fe N doped graphene K. Parvez et al. ACS Nano 2012, 6, 9541-9550.
- 17. Update researchs Kinetic activities of the main Pt-based electrocatalyst systems at 0.9 V vs. Reversible Hydrogen Electrode (RHE): (a) Activities are measured by rotating disc electrode (RDE) and (b) Activities are measured in membrane electrode assemblies (MEAs) at 80 °C and 150 kPa saturated O2. Reprinted with permission from Ref. [9]. Copyright 2012, Nature Publishing Group.
- 18. TEM-HRTEM micrographs and their corresponding particle size distribution (histograms were fitted using the log-normal function) of the nanostructured AuPt (20 wt%) supported on (a) Vulcan XC 72R and (b) Ketjenblack EC-600JD. (a) Reprinted and adapted with permission from Ref. [103]. Copyright 2014, John Wiley & Sons, Inc. (b) Reprinted and adapted with permission from Ref. [102]. Copyright 2014, John Wiley & Sons, Inc. Update research
- 19. Schematic representation of the oxygen reduction reaction (ORR) mechanism by direct pathway (A: adsorption parallel to the surface) and indirect pathway (B: adsorption perpendicular to the surface). Reprinted and adapted with permission from Ref. [125]. Copyright 1997, Elsevier. Update research
- 20. (a) Koutecky–Levich and (b) jk−1 plot for the determination of jL. Inset in (b) shows the Tafel plots: data are extracted from ORR after the durability test in 0.1 M HClO4. (c) Comparison of jk at 0.9 V vs. RHE in 0.1 M HClO4 and 0.1 M NaOH. Update research
- 21. A novel, hollow Fe–N–C hybrid nanostructures that act as active and durable electrocatalytic materials J.H. Lee et al. / Inorganica Chimica Acta 422 (2014) 3–7 Update research
- 22. 2NH3 3H2 + N2 △H = 46kJ/mol Ammonia decomposition Hien’s Research objective
- 23. 23
Editor's Notes
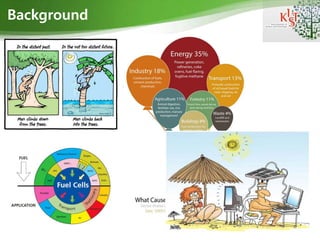
- An issue is that in the distant past man climbs down from the trees. Nevertheless in the not too far future man will climbs back into the trees. Because of the global warming and green house effects that make our planet melt down and increase the sea level year by year. These are several factor that cause GHG emission but as you can see the biggest balloon is contributed for energy. Oil and gasoline price become higher and higher, consume fossil fuel leads to gas house effects. There is a great energy challenge for our living standard to find alternative energy sources and compare with exiting fuel systems, the hydrogen FC technology is a promising solution to these environmental challenges. FC can apply on a series of fields such as transport, stationary, portable.
- It is higher need to focus to change the petroleum dependence issue and to reduce carbon dioxide emissions. This is an stark example of hydrogen fuel cell application. Base on hydrogen fuel and Oxygen reduction reaction generate energy with free CO2 emissions only water and heat. Do you know how to improve the ORR activity? The answer is we need to improve the catalytic performance on cathode rxn
- So that today I would like to make a review on a study titled:… is was public on science journal one of the highest impact factor or all science journals. Event thought it quite old 8 years ago but it make a significant contribution for ORR study as well as PEMFC applications. At that time, this new variation of the platinum–nickel alloy is the most active oxygen-reducing catalyst ever reported.
- 2. Even on the best catalysts like Pt, losses due to slow reaction kinetics lead to an efficiency loss of around 20%.
- Rather than use a trial-and-error or combinatorial approach
- A combination of in situ and ex situ surface-sensitive probes and density functional theory (DFT) calculations was used to study ORR on Pt3Ni(hkl) single-crystal surfaces. To answer questions… a series of charactertization was use they are…
- That avoid the bonding Pt-OH so increase active site and boosts cat performance
- The Pt3Ni(111) surface has an unusual electronic structure (d-band center position) and arrangement of surface atoms in the near-surface region. its near-surface layer exhibits a highly structured compositional oscillation in the outermost and third layers, which are Pt-rich, and in the second atomic layer, which is Ni-rich. This causes a weakening of the bonds between the Pt surface atoms and the OH– molecules. The weakening increases the number of active sites available for O2 adsorption. As the kinetics of O2 reduction are determined by the number of free Pt sites available for the adsorption of O2, the intrinsic catalytic activity at the fuel-cell-relevant potentials (E > 0.8 V) has been found to be ten times more active than the corresponding Pt(111) surface. The observed catalytic activity for the ORR on Pt3Ni(111) is the highest ever observed on cathode catalysts, including the Pt3Ni(100) and Pt3Ni(110) surfaces.