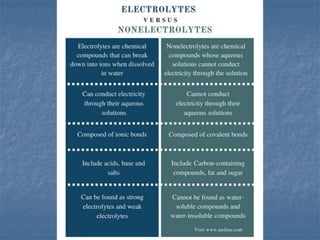
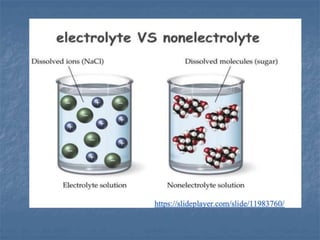
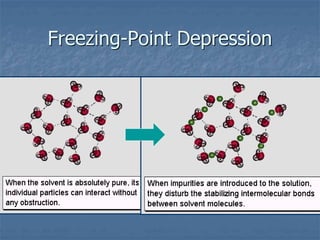
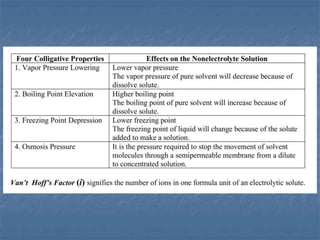
The document discusses four colligative properties of solutions:
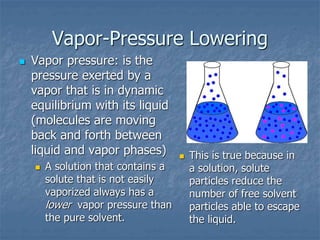
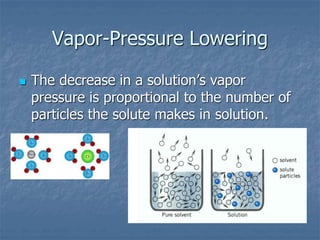
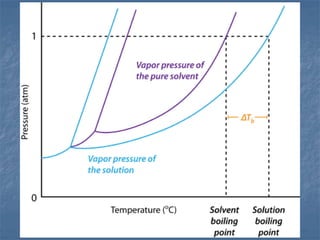
1) Vapor pressure lowering - Adding a solute lowers the vapor pressure of the solvent by reducing the number of free solvent particles that can evaporate.
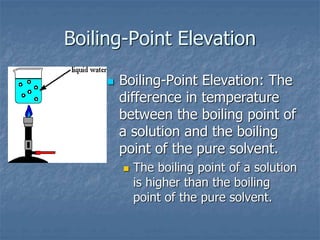
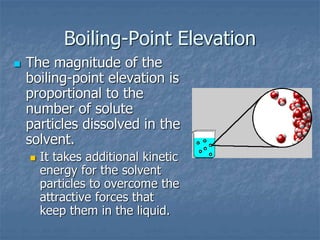
2) Boiling point elevation - A solution's boiling point is higher than the pure solvent's due to the vapor pressure lowering effect requiring more energy for evaporation.
3) Freezing point depression - A solution's freezing point is lower than the pure solvent's because the solute disrupts the formation of an orderly ice crystal lattice, requiring more energy be removed for freezing.
4) Osmotic pressure - The document does not provide details about osmotic pressure.