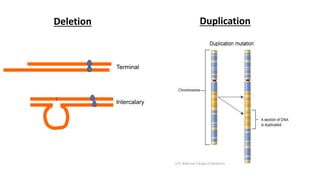
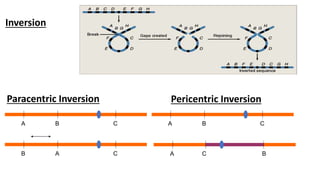
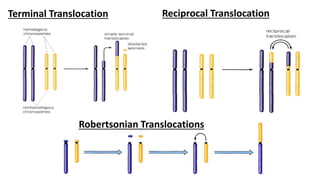
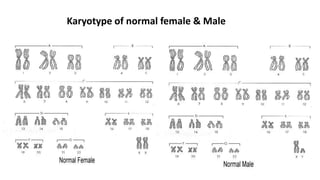
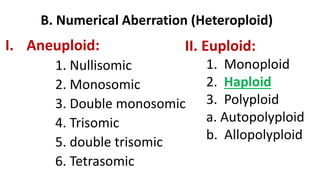
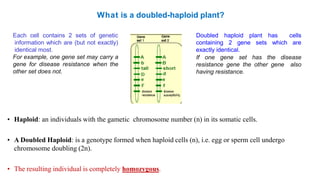
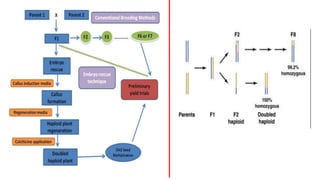
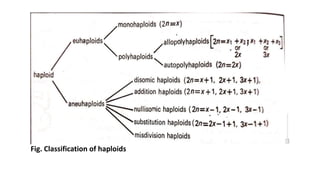
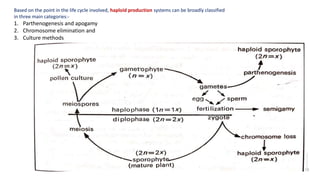
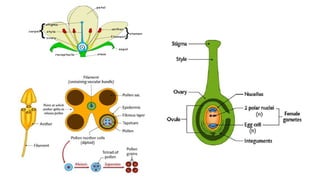
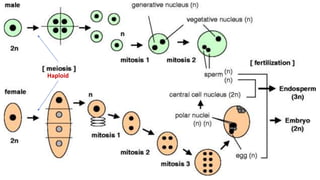
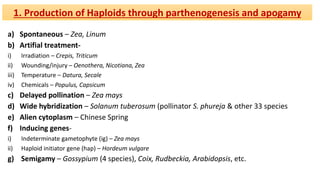
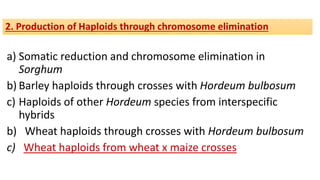
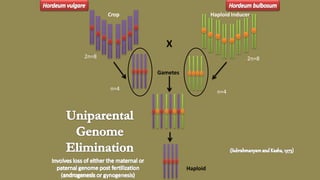
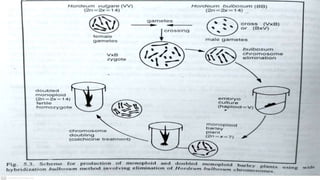
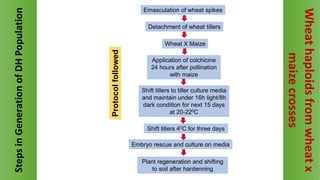
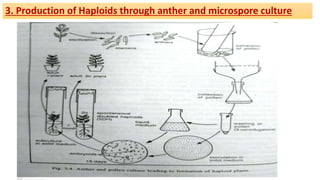
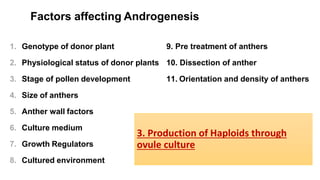
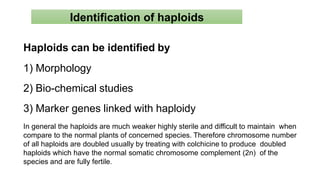
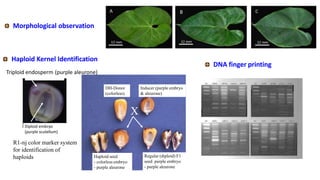
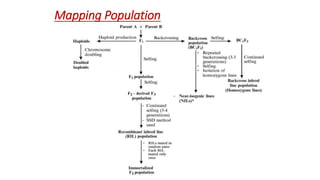
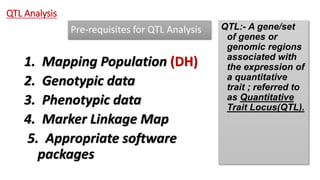
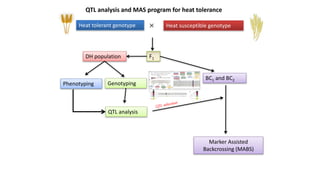
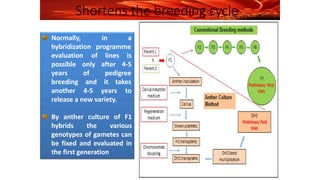
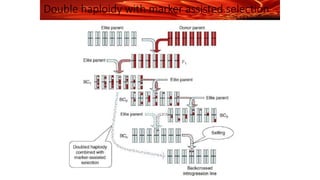
The document discusses haploidy and doubled haploid technology. It describes three main methods for producing haploids: parthenogenesis and apogamy, chromosome elimination, and culture methods. Haploids are useful in plant breeding as they allow for the rapid creation of fully homozygous lines, shortening breeding cycles. Doubled haploids can be used to generate mapping populations for QTL analysis and marker-assisted selection can then be used to introgress traits of interest.