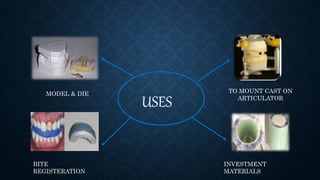
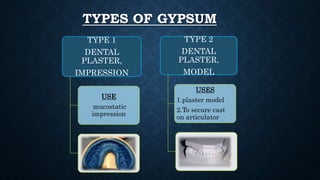
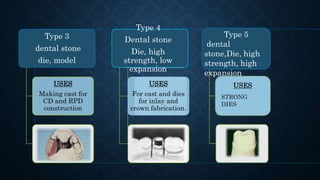
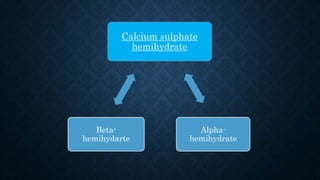
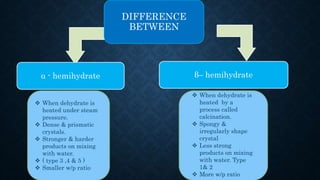
Gypsum, primarily composed of calcium sulfate dihydrate, is used in dentistry to create models and dies for various applications. There are different types of gypsum products, such as dental plaster and dental stone, which vary in strength, porosity, and mixing ratios, affecting their suitability for different dental tasks. While gypsum materials are inexpensive and capable of reproducing detail, they are brittle and can fracture under stress, particularly when used with certain impression materials.