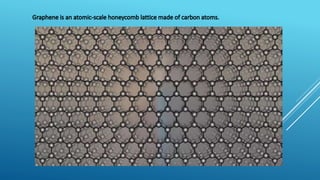
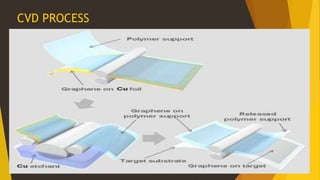
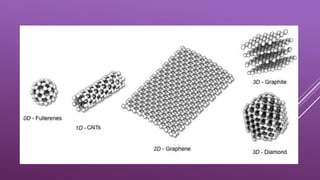
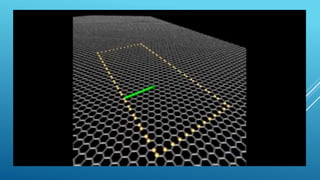
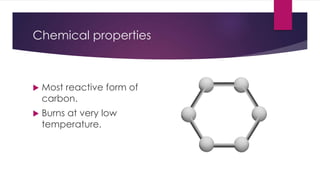
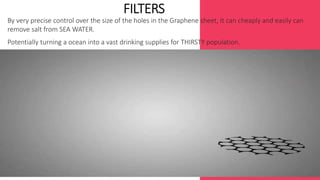
This document summarizes the properties and potential applications of graphene. Graphene is an extremely thin material made of carbon that is very strong, conductive, and flexible. It has potential uses in electronics, composite materials for vehicles, desalination, biomedical devices, and antibacterial applications. However, large-scale production of graphene remains challenging and it lacks the ability to act as a transistor, limiting its use in digital electronics currently dominated by silicon.