Putting a Personalized Colorectal Cancer Treatment Algorithm Into Practice: Navigating Practicalities in the Era of Molecularly Defined Care
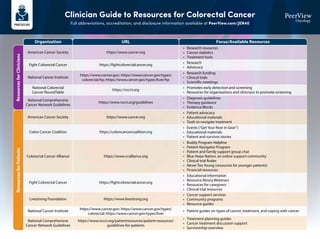
- 1. Clinician Guide to Resources for Colorectal Cancer Full abbreviations, accreditation, and disclosure information available at PeerView.com/JEN40 Organization URL Focus/Available Resources Resources for Clinicians Resources for Patients American Cancer Society https://www.cancer.org • Research resources • Cancer statistics • Treatment tools American Cancer Society https://www.cancer.org • Patient advocacy • Educational materials • Tools to navigate treatment Colon Cancer Coalition https://coloncancercoalition.org • Events (“Get Your Rear in Gear”) • Educational materials • Patient and survivor stories Livestrong Foundation https://www.livestrong.org • Cancer support services • Community programs • Resource guides Colorectal Cancer Alliance https://www.ccalliance.org • Buddy Program Helpline • Patient Navigator Program • Patient and family support group chat • Blue Hope Nation, an online support community • Clinical trial finder • Never Too Young (resources for younger patients) • Financial resources Fight Colorectal Cancer https://fightcolorectalcancer.org • Research • Advocacy Fight Colorectal Cancer https://fightcolorectalcancer.org • Educational information • Resource library Webinars • Resources for caregivers • Clinical trial resources National Comprehensive Cancer Network Guidelines • Treatment planning guides • Cancer treatment discussion support • Survivorship overview https://www.nccn.org/patientresources/patient-resources/ guidelines-for-patients National Cancer Institute https://www.cancer.gov; https://www.cancer.gov/types/ colorectal; https://www.cancer.gov/types/liver • Patient guides on types of cancer, treatment, and coping with cancer National Cancer Institute https://www.cancer.gov; https://www.cancer.gov/types/ colorectal/hp; https://www.cancer.gov/types/liver/hp • Research funding • Clinical trials • Scientific meetings National Colorectal Cancer RoundTable https://nccrt.org • Promotes early detection and screening • Resources for organizations and clinicians to promote screening National Comprehensive Cancer Network Guidelines https://www.nccn.org/guidelines • Diagnosis guidelines • Therapy guidance • Evidence Blocks
- 2. Colorectal Cancer: Current and Emerging Treatment Options Full abbreviations, accreditation, and disclosure information available at PeerView.com/JEN40 Selected Clinical Trials NCT Number NCT05004350 Encorafenib + cetuximab vs irinotecan/cetuximab or FOLFIRI/cetuximab BRAF V600E–mutant metastatic CRC Phase 2, not yet recruiting NAUTICALCRC NCT03693170 Encorafenib + binimetinib + cetuximab BRAF V600E–mutant metastatic CRC Phase 2; active, not recruiting ANCHOR CRC NCT04607421 Encorafenib + cetuximab ± CT vs standard of care therapy with a safety lead-in of encorafenib + cetuximab + CT Previously untreated BRAF V600E–mutant metastatic CRC Phase 3, recruiting BREAKWATER NCT04673955 Encorafenib + cetuximab BRAF V600E–mutant metastatic CRC Recruiting BERING CRC Study Title Condition Regimen Phase/ Recruitment Status Encorafenib/Cetuximab NCT Number NCT03524820 NCT03446157 NCT04515394 NCT01910610 NCT02713373 Cetuximab Cetuximab + palbociclib Colorectal neoplasms FOLFIRI/cetuximab, followed by oxaliplatin-based CT with bevacizumab vs OPTIMOX/bevacizumab, followed by irinotecan-based CT with bevacizumab, followed by anti-EGFR mAb with or without irinotecan Cetuximab + pembrolizumab Metastatic CRC Metastatic CRC Tepotinib + cetuximab Metastatic CRC Metastatic CRC that cannot be removed by surgery Phase 2, recruiting Phase 2, recruiting Phase 2, recruiting Phase 3, recruiting Phase 1/2; active, not recruiting Cetuximab Therapy for Third-Line Rechallenge in Metastatic Colorectal Cancer Palbociclib and Cetuximab in Metastatic Colorectal Cancer PERSPECTIVE STRATEGIC-1 Cetuximab and Pembrolizumab in Treating Patients With Colorectal Cancer That Is Metastatic or Cannot Be Removed by Surgery Study Title Condition Regimen Phase/ Recruitment Status Cetuximab
- 3. Colorectal Cancer: Current and Emerging Treatment Options Full abbreviations, accreditation, and disclosure information available at PeerView.com/JEN40 Selected Clinical Trials NCT03658772 NCT04991948 MSI-stable CRC Unresectable metastatic CRC Grapiprant + pembrolizumab CYAD-101 with FOLFOX infusion administered concurrently followed by pembrolizumab Phase 1, recruiting Phase 1, not yet recruiting Grapiprant and Pembrolizumab in Patients With Advanced or Progressive MSS Colorectal Cancer Phase 1b Study to Evaluate the Addition of a Pembrolizumab Treatment After Treatment With CYAD-101 With a FOLFOX Preconditioning in Metastatic Colorectal Cancer Patients NCT Number Study Title Condition Regimen Phase/ Recruitment Status NCT03374254 NCT02437071 Pembrolizumab + binimetinib vs pembrolizumab + mFOLFOX7 vs pembrolizumab + mFOLFOX7 + binimetinib vs pembrolizumab + FOLFIRI vs pembrolizumab + FOLFIRI + binimetinib Pembrolizumab + radiotherapy vs pembrolizumab + ablation Metastatic CRC Metastatic CRC Phase 1; active, not recruiting Phase 2; active, not recruiting MK-3475-651 Assess the Efficacy of Pembrolizumab Plus Radiotherapy or Ablation in Metastatic Colorectal Cancer Patients NCT04854434 Selinexor ± pembrolizumab Metastatic CRC Phase 2, recruiting A Study to Evaluate the Safety and Efficacy of Selinexor With or Without Pembrolizumab Versus Standard of Care in Previously Treated Metastatic Colorectal Cancer With RAS Mutations Pembrolizumab NCT Number NCT03520946 Ramucirumab + TAS102 vs TAS102 CT-refractory advanced metastatic CRC Phase 3, recruiting RAMTAS NCT03798626 Metastatic colorectal, gastroesophageal, and renal cancers Phase 1, recruiting Gevokizumab With Standard of Care Anticancer Therapies for Metastatic Colorectal, Gastroesophageal, and Renal Cancers Gevokizumab + modified FOLFOX6 + bevacizumab (first-line tx); gevokizumab + FOLFIRI + bevacizumab (second-line tx) Study Title Condition Regimen Phase/ Recruitment Status Ramucirumab
- 4. Colorectal Cancer: Current and Emerging Treatment Options Full abbreviations, accreditation, and disclosure information available at PeerView.com/JEN40 Selected Clinical Trials NCT Number NCT04776148 Lenvatinib + pembrolizumab vs regorafenib or TAS102 Metastatic CRC Phase 3, recruiting LEAP-017 NCT04965870 TAS102 CT-refractory metastatic CRC Recruiting RETRO-TAS NCT04073615 TAS102 ± rivoceranib Metastatic CRC Phase 1/2, recruiting Phase 1b/2 Study of Rivoceranib and Trifluridine/Tipiracil for Metastatic Colorectal Cancer NCT04511039 TAS102 + talazoparib Locally advanced or metastatic CRC or gastroesophageal cancer Phase 1, recruiting Trifluridine/Tipiracil and Talazoparib for the Treatment of Patients With Locally Advanced or Metastatic Colorectal or Gastroesophageal Cancer NCT03223779 TAS102 + radiation therapy Hepatic metastases from CRC Phase 1/2, recruiting Study of TAS-102 Plus Radiation Therapy for the Treatment of the Liver in Patients With Hepatic Metastases From Colorectal Cancer NCT04737187 TAS102 ± bevacizumab Refractory metastatic CRC Phase 3, recruiting SUNLIGHT Study Title Condition Regimen Phase/ Recruitment Status TAS102 NCT03635021 CRC Phase 3, recruiting CR-SEQUENCE FOLFOX + panitumumab followed by FOLFIRI + bevacizumab vs FOLFOX + bevacizumab followed by FOLFIRI + panitumumab NCT Number Study Title Condition Regimen Phase/ Recruitment Status Bevacizumab
- 5. Colorectal Cancer: Current and Emerging Treatment Options Full abbreviations, accreditation, and disclosure information available at PeerView.com/JEN40 Selected Clinical Trials NCT Number NCT02301962 Panitumumab Metastatic CRC Phase 4, recruiting Phase IV Panitumumab Study in Indian Subjects With Metastatic Colorectal Cancer NCT03043950 Panitumumab RAS wild-type metastatic CRC Active, not recruiting VALIDATE NCT03751176 FOLFIRI ± panitumumab RAS wild-type metastatic CRC Phase 2; active, not recruiting BEYOND NCT03584711 FOLFOX + panitumumab Metastatic CRC Phase 2, recruiting OPTIPRIME NCT01328171 FOLFOXIRI ± panitumumab Nonresectable, RAS wild-type metastatic CRC Phase 2; active, not recruiting VOLFI NCT01312857 Panitumumab Resected, RAS wild-type metastatic CRC Phase 2; active, not recruiting Study of Hepatic Arterial Infusion With Intravenous Irinotecan, 5-FU, and Leucovorin With or Without Panitumumab, in Patients With Wild-Type RAS Who Have Resected Hepatic Metastases From Colorectal Cancer NCT01991873 Maintenance CT ± panitumumab RAS wild-type metastatic CRC Phase 2; active, not recruiting PanaMa Study Title Condition Regimen Phase/ Recruitment Status Panitumumab
- 6. Current Clinical Guidelines for Therapy Selection and Molecular Testing in mCRC Full abbreviations, accreditation, and disclosure information available at PeerView.com/JEN40 NCCN Clinical Practice Guidelines: First-Line Therapies for Colon Cancer1 Patient Appropriate for Intensive Therapy? See subsequent therapy See subsequent therapy Yes No NCCN Clinical Practice Guidelines: Second- and Third-Line Therapies for Colon Cancer1 • FOLFOX ± bevacizumab • CAPEOX ± bevacizumab • FOLFOX + cetuximab or panitumumab (KRAS/NRAS/BRAF WT and left-sided tumors only) • FOLFIRI ± bevacizumab • FOLFIRI + cetuximab or panitumumab (KRAS/NRAS/BRAF WT and left-sided tumors only) • FOLFOXIRI ± bevacizumab • Pembrolizumab or nivolumab ± ipilimumab (dMMR/MSI-H only) • FOLFIRI or irinotecan • FOLFIRI + bevacizumab (preferred) or ziv-aflibercept or ramucirumab • Irinotecan + bevacizumab (preferred) or ziv-aflibercept or ramucirumab or • FOLFIRI + cetuximab or panitumumab (KRAS/NRAS/BRAF WT only) • Irinotecan + cetuximab or panitumumab (KRAS/NRAS/BRAF WT only) • Encorafenib + cetuximab or panitumumab (BRAF V600 mutation positive) or • Nivolumab ± ipilimumab or pembrolizumab (dMMR/MSI-H only) • Trastuzumab + (pertuzumab or lapatinib) or trastuzumab deruxtecan (HER2-amplified and RAS and BRAF WT) • Irinotecan + (cetuximab or panitumumab) (KRAS/NRAS/BRAF WT only) • Regorafenib • Trifluridine + tipiracil ± bevacizumab • Nivolumab ± ipilimumab or pembrolizumab (dMMR/MSI-H only) • Trastuzumab + (pertuzumab or lapatinib) or trastuzumab deruxtecan (HER2-amplified and RAS and BRAF WT) • Regorafenib • Trifluridine + tipiracil ± bevacizumab • Nivolumab ± ipilimumab or pembrolizumab (dMMR/MSI-H only) • Trastuzumab + (pertuzumab or lapatinib) or trastuzumab deruxtecan (HER2-amplified and RAS and BRAF WT) • Infusional 5-FU ± leucovorin ± bevacizumab • Capecitabine ± bevacizumab • Cetuximab or panitumumab (category 2B) (KRAS/NRAS/BRAF WT and left-sided tumors only) • Nivolumab or pembrolizumab (dMMR/MSI-H only) • Nivolumab + ipilimumab (dMMR/MSI-H only) (category 2B) • Trastuzumab + (pertuzumab or lapatinib) or trastuzumab deruxtecan (HER2-amplified and RAS and BRAF WT) Previous oxaliplatin-based therapy without irinotecan See subsequent therapy
- 7. Current Clinical Guidelines for Therapy Selection and Molecular Testing in mCRC Full abbreviations, accreditation, and disclosure information available at PeerView.com/JEN40 Integrating New Clinical Data Into the CRC Treatment Algorithm What if it’s right-sided MSI-H or BRAF V600E–mutant CRC? 1L recommendation 3L recommendation 2L recommendation What if the tumor progresses on 2L therapy? What if the tumor progresses on previous therapy? Pembrolizumab or nivolumab ± ipilimumab (dMMR/MSI-H only) Encorafenib/cetuximab + binimetinib triplet (BRAF V600E only) Cytotoxic treatment + bevacizumab/anti-EGFR antibody TAS102 + bevacizumab Regorafenib FOLFIRI + bevacizumab (preferred) or ziv-aflibercept or ramucirumab FOLFOX + bevacizumab FOLFIRI/irinotecan + cetuximab or panitumumab Encorafenib + cetuximab or panitumumab (BRAF V600 mutation) Supported by KEYNOTE-177, CheckMate -142, CALGB/SWOG 80405, ANCHOR CRC2-6 Supported by RECOURSE, REARRANGE10,11 Supported by BEACON CRC, CheckMate -142, GARNET2,7-9 David presents with asymptomatic mCRC with wild-type KRAS and NRAS • Aged 63 years • CBC: Hb 11 g/dL; PLT 172 x 109 /L • 4 cm retroperitoneal lymphadenopathy • ECOG PS 1
- 8. Current Clinical Guidelines for Therapy Selection and Molecular Testing in mCRC Full abbreviations, accreditation, and disclosure information available at PeerView.com/JEN40 NCCN Clinical Practice Guidelines for Molecular Testing in CRC All patients diagnosed with CRC should be tested for the following mutations NCCN Guidelines: Microsatellite Instability or Mismatch Repair Testing1 RAS mutations • NRAS • KRAS BRAF dMMR/ MSI-H status Universal mismatch repair (MMR)a or microsatellite instability (MSI) testing is recommended in all newly diagnosed patients with colon cancer MMR or MSI testing should be performed only in CLIA-approved laboratories Testing for MSI may be accomplished by PCR or a validated NGS panel—the latter especially in patients with metastatic disease who require genotyping of RAS and BRAF The presence of BRAF V600E mutation or MLH1 promoter methylation is consistent with sporadic cancer Abnormal MLH1 IHC should be followed by tumor testing for BRAF V600E mutation or MLH1 promoter methylation IHC refers to staining tumor tissue for protein expression of the 4 MMR genes known to be mutated in LS (MLH1, MSH2, MSH6, and PMS2) a IHC for MMR and DNA analysis for MSI are different assays and measure different biological effects caused by deficient MMR function.
- 9. Current Clinical Guidelines for Therapy Selection and Molecular Testing in mCRC Full abbreviations, accreditation, and disclosure information available at PeerView.com/JEN40 1. NCCN Clinical Practice Guidelines in Oncology. Colon Cancer. Version 3.2021. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. 2. Andre T et al. 2021 American Society of Clinical Oncology Annual Meeting (ASCO 2021). Abstract 3500. 3. Lenz H-J et al. ASCO 2020. Abstract 4040. 4. Venook A et al. European Society for Medical Oncology Congress 2016 (ESMO 2016). Oral presentation. 5. Van Cutsem E et al. ESMO World Congress on Gastrointestinal Cancer 2021 (ESMO World GI 2021). Abstract O-10. 6. https://clinicaltrials.gov/ct2/show/ NCT03693170. 7. Kopetz S, Van Cutsem E et al. N Engl J Med. 2019;381:1632-1643. 8. https://clinicaltrials.gov/ct2/show/NCT02928224. 9. Berton D et al. ASCO 2021. Abstract 2564. 10. Mayer RJ et al. N Engl J Med. 2015;372:1909-1919. 11. Argilés G et al. ESMO World GI 2019. Abstract O-026. 12. Van Cutsem E et al. Ann Oncol. 2016;27:1386-1422. Many Drivers for First-Line Treatment Are Also Valid in Later Line12 Patient and treatment characteristics become even more relevant in later lines Genetic Mutations Affect Treatment Selection1 Patients with RAS mutations do not respond to anti-EGFR agents Patients with BRAF V600E mutations respond poorly to anti-EGFR agents unless given with a BRAF inhibitor Checkpoint inhibitors are effective for dMMR/MSI-H tumors Tumor Characteristics Patient Characteristics Treatment Characteristics • Clinical presentation – Tumor burden – Tumor localization • Tumor biology • RAS mutation status • BRAF mutation status • Age • Performance status • Organ function • Comorbidities; patient attitude, expectation, and preference • Toxicity profile • Flexibility of treatment administration • Socioeconomic factors • Quality of life
