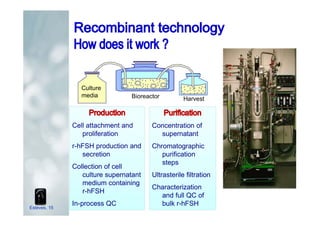
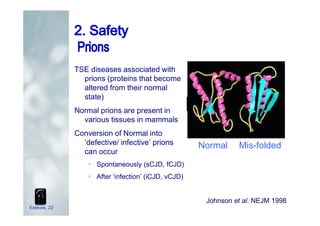
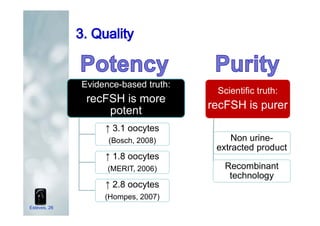
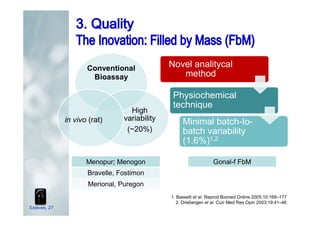
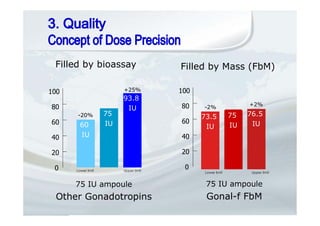
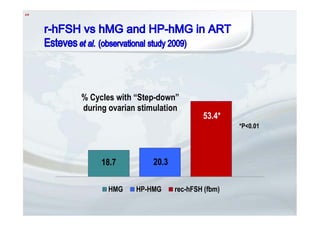
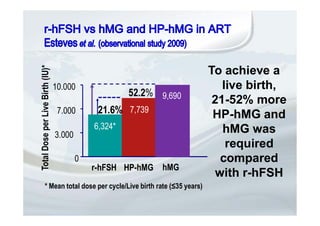
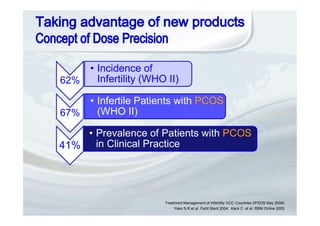
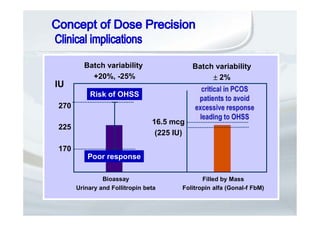
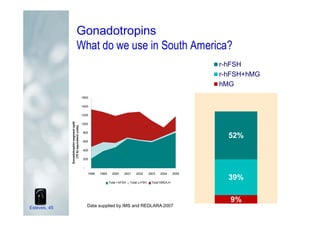
1. The document discusses the evolution of gonadotropins used in assisted reproduction, including their development from urinary sources to recombinant products.
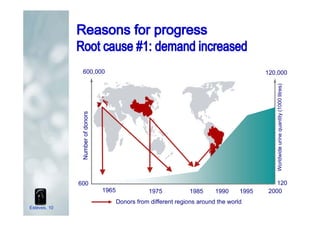
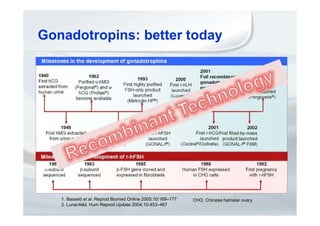
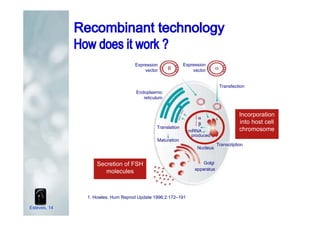
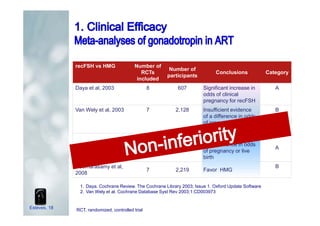
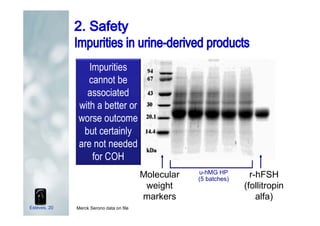
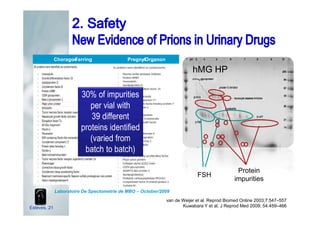
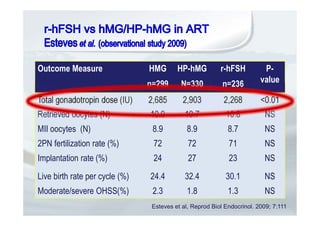
2. It describes how recombinant gonadotropins provide higher purity and more consistent dosage compared to earlier urinary products, with clinical trials demonstrating similar or improved outcomes.
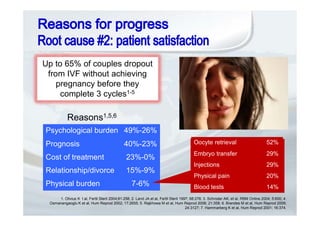
3. The use of recombinant gonadotropins can offer benefits to patients through features such as pre-filled pens, which may improve compliance and reduce stress associated with treatment.