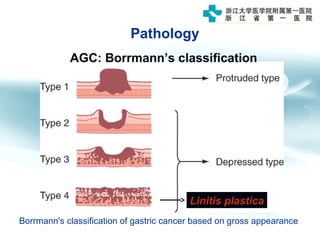
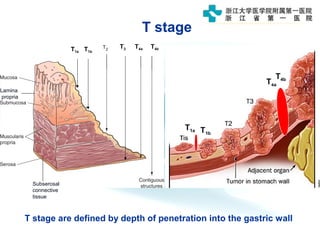
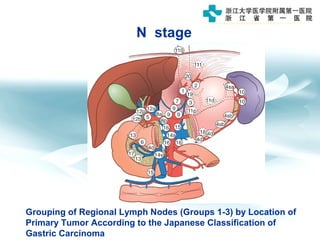
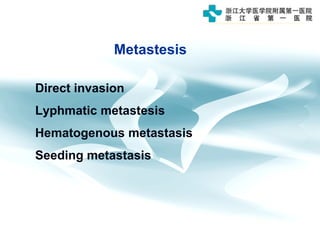
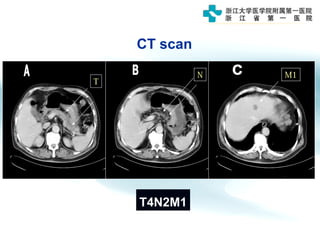
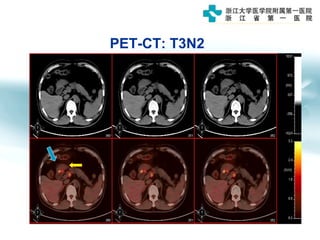
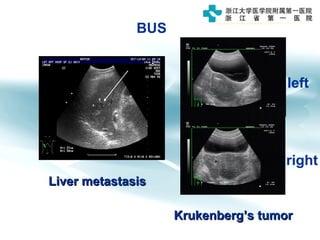
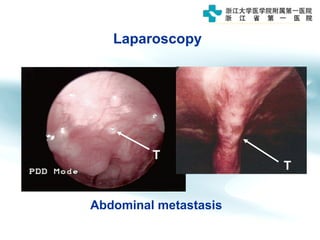
1) Gastric carcinoma is the third leading cause of cancer death worldwide, with highest incidence in East Asia and parts of South America.
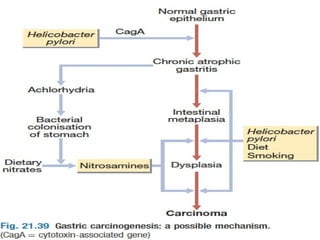
2) Risk factors include H. pylori infection, smoking, diet high in salted/preserved foods, and family history of gastric cancer.
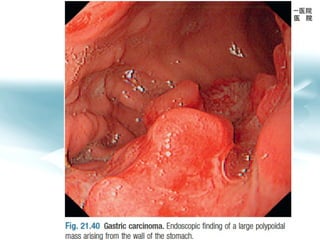
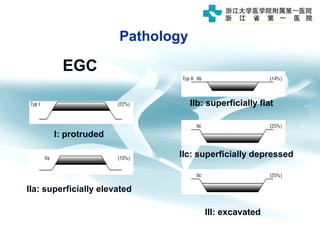
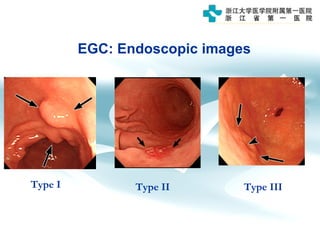
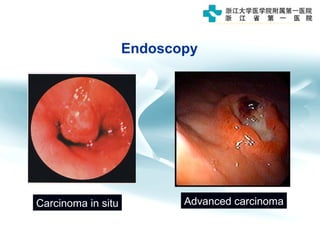
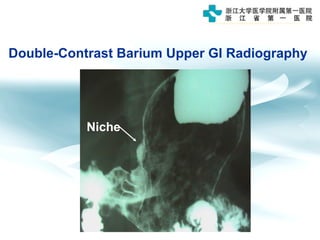
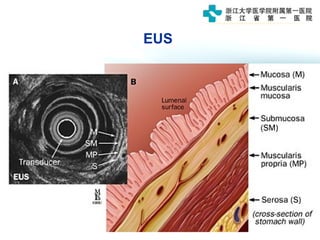
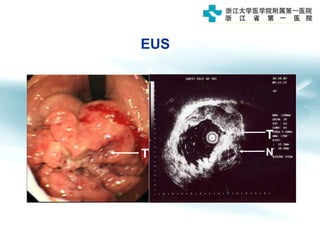
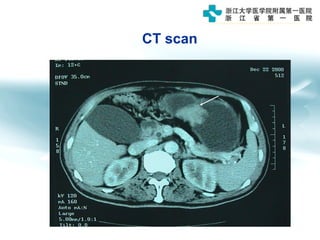
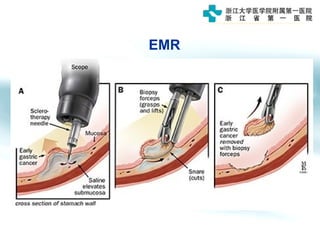
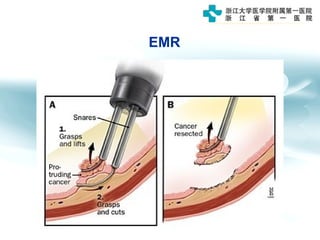
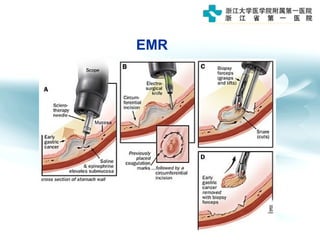
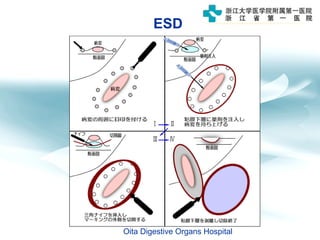
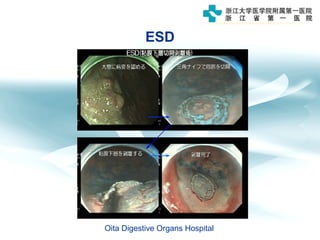
3) Early detection through endoscopy in dyspeptic patients over 50 years old or with red flags can improve outcomes, as resection allows for potential cure in early gastric cancer confined to mucosa or submucosa.