This document provides information on gastric carcinoma, including:
1. It describes the classification, epidemiology, risk factors, pathogenesis, histology, staging, clinical features, investigations, and management of gastric adenocarcinoma.
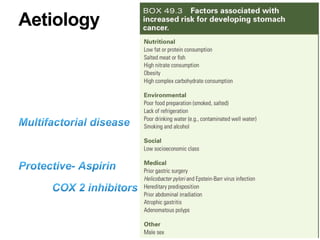
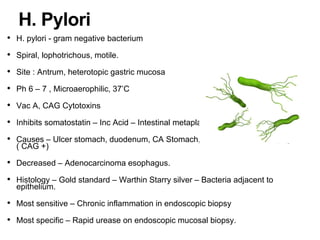
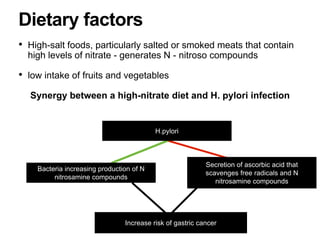
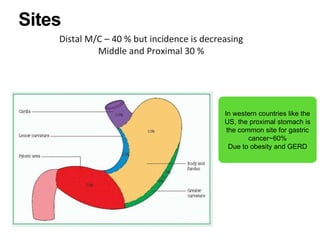
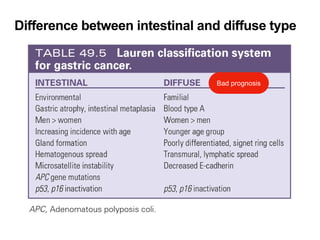
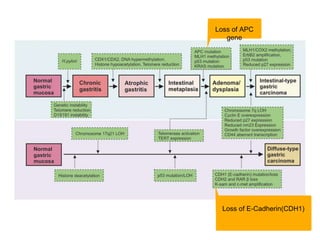
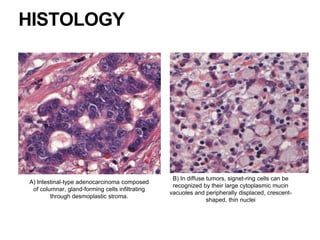
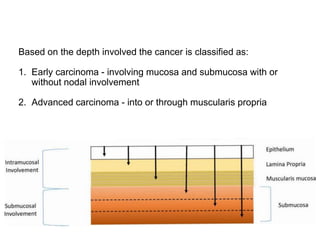
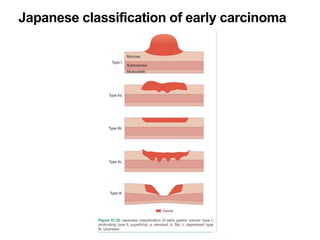
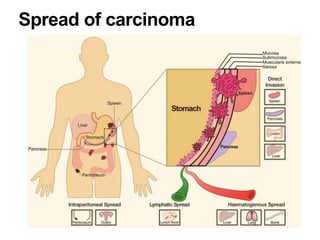
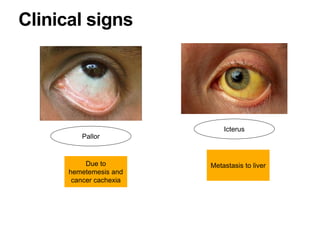
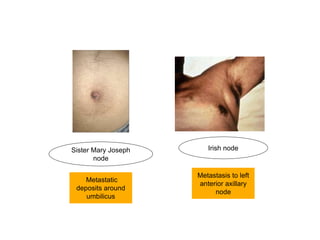
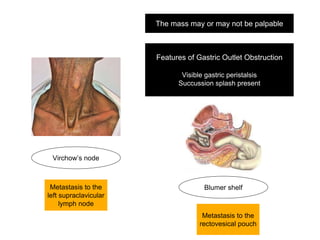
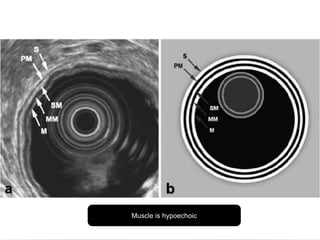
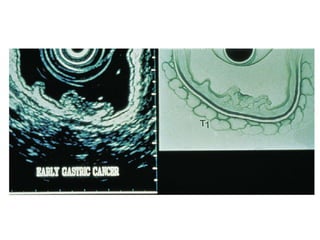
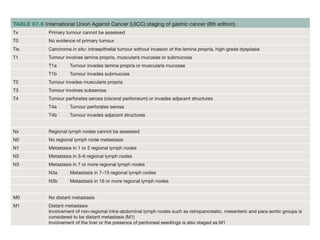
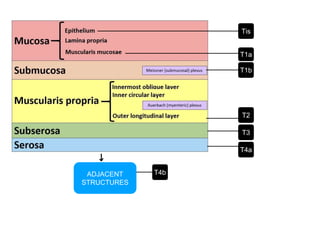
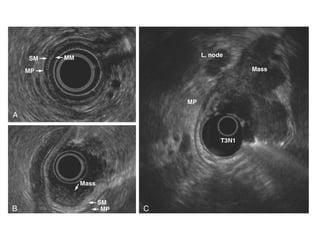
2. The main risk factors include H. pylori infection, dietary nitrites, genetic mutations, and polyps. Gastric adenocarcinoma is classified based on cell type, location, depth of invasion, and metastasis.
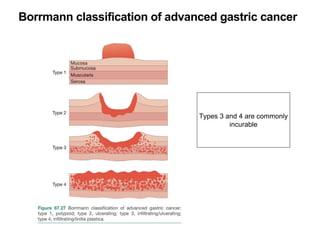
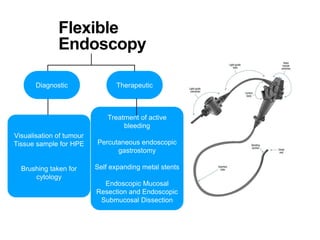
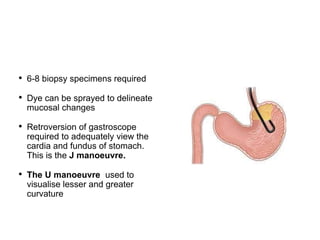
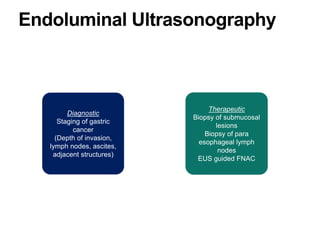
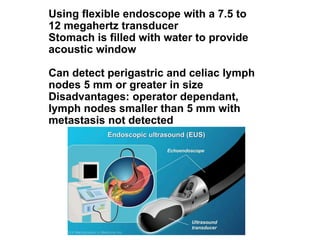
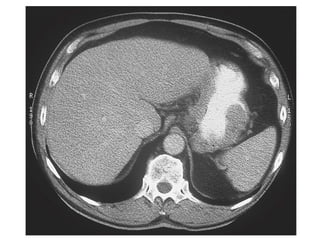
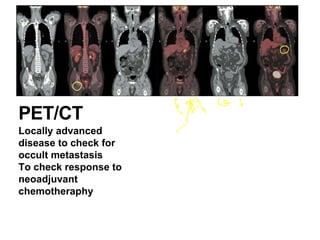
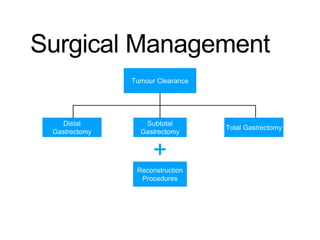
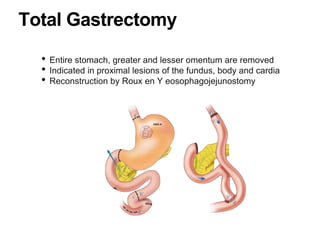
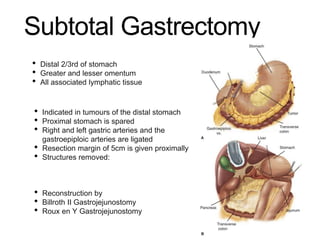
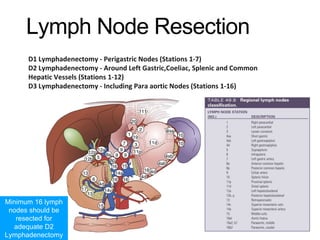
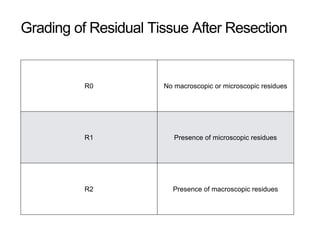
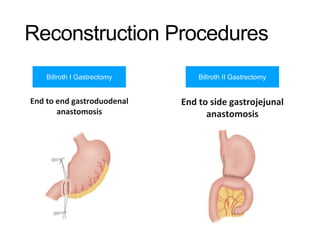
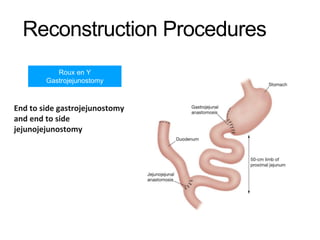
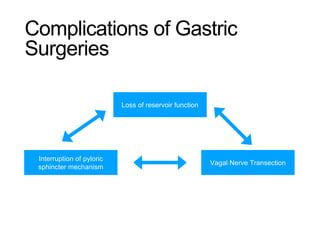
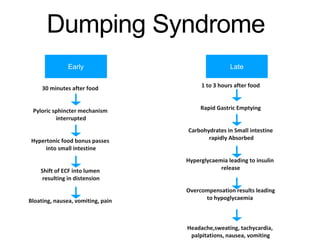
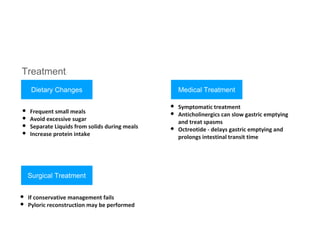
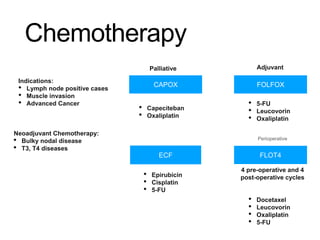
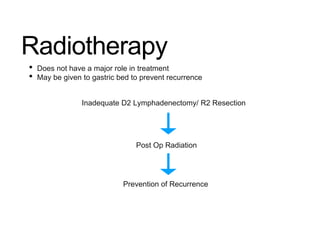
3. Management involves endoscopic resection for early cancers, while advanced cancers are treated with surgery such as gastrectomy, with or without chemotherapy and radiotherapy. Complications and palliative care are also discussed.