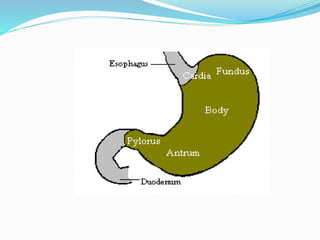
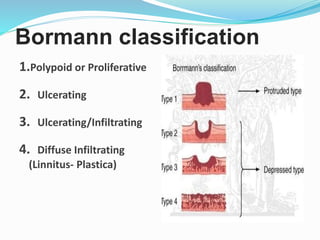
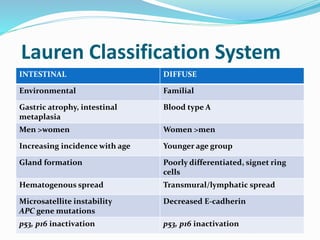
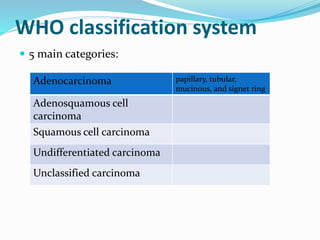
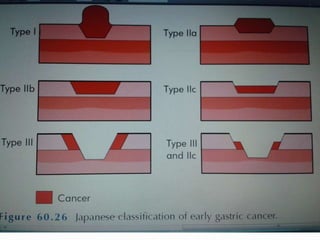
This document discusses gastric cancer, including its incidence, risk factors, pathogenesis, clinical presentation, diagnostic evaluation, staging, and treatment approaches. Some key points include:
- Gastric cancer has a poor prognosis with only 20% 5-year survival. Early diagnosis is key.
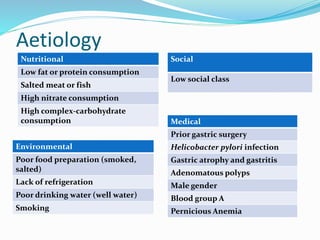
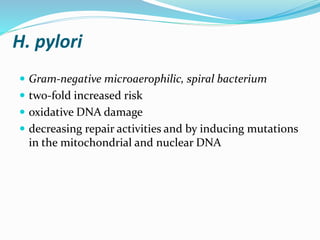
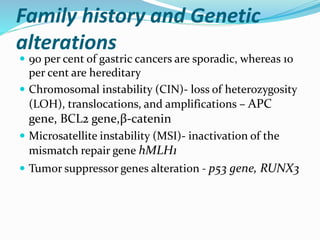
- Risk factors include H. pylori infection, smoking, low socioeconomic status, and diets high in salt/preserved foods.
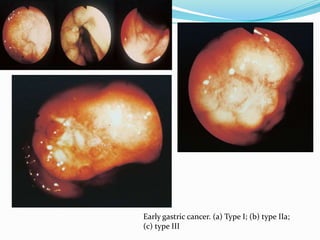
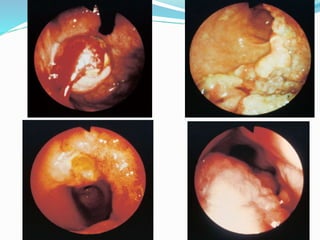
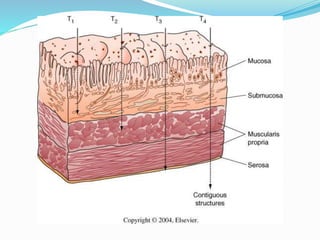
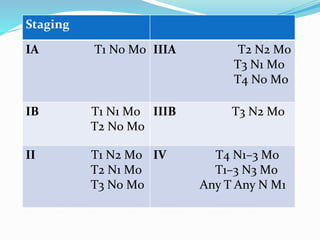
- Diagnosis involves endoscopy with biopsy. Staging evaluates tumor invasion and metastasis using CT, PET, and laparoscopy.
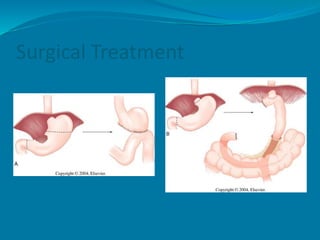
- Surgery offering total or subtotal gastrectomy is the only curative option, while chemotherapy and radiation are palliative.