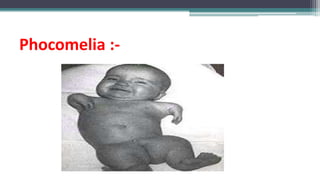
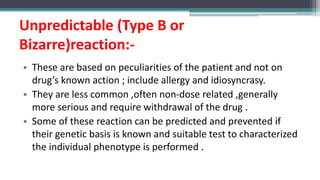
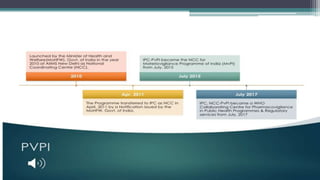
This document provides an overview of pharmacovigilance and the Pharmacovigilance Program of India (PvPI). It defines pharmacovigilance as the science of detecting, assessing, understanding, and preventing adverse effects of medicines. The document outlines the historical events that led to the development of pharmacovigilance, including the sulfanilamide and thalidomide disasters. It describes the vision, mission, aims, and objectives of PvPI in India to improve patient safety related to medicine use. Key aspects of PvPI covered include adverse drug reaction reporting processes and the expansion of PvPI to include additional medical areas over time.