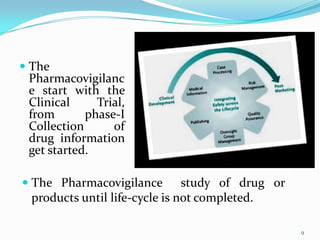
Pharmacovigilance is the science of monitoring the safety of medicines. It plays a key role in identifying adverse drug reactions and preventing harm to patients. The goal of pharmacovigilance is to monitor drug safety, identify health risks, and prevent harm through careful tracking of side effects from clinical trials through a drug's entire lifecycle on the market. Major organizations like the FDA, EMEA, and WHO work to coordinate pharmacovigilance efforts internationally and protect public health.