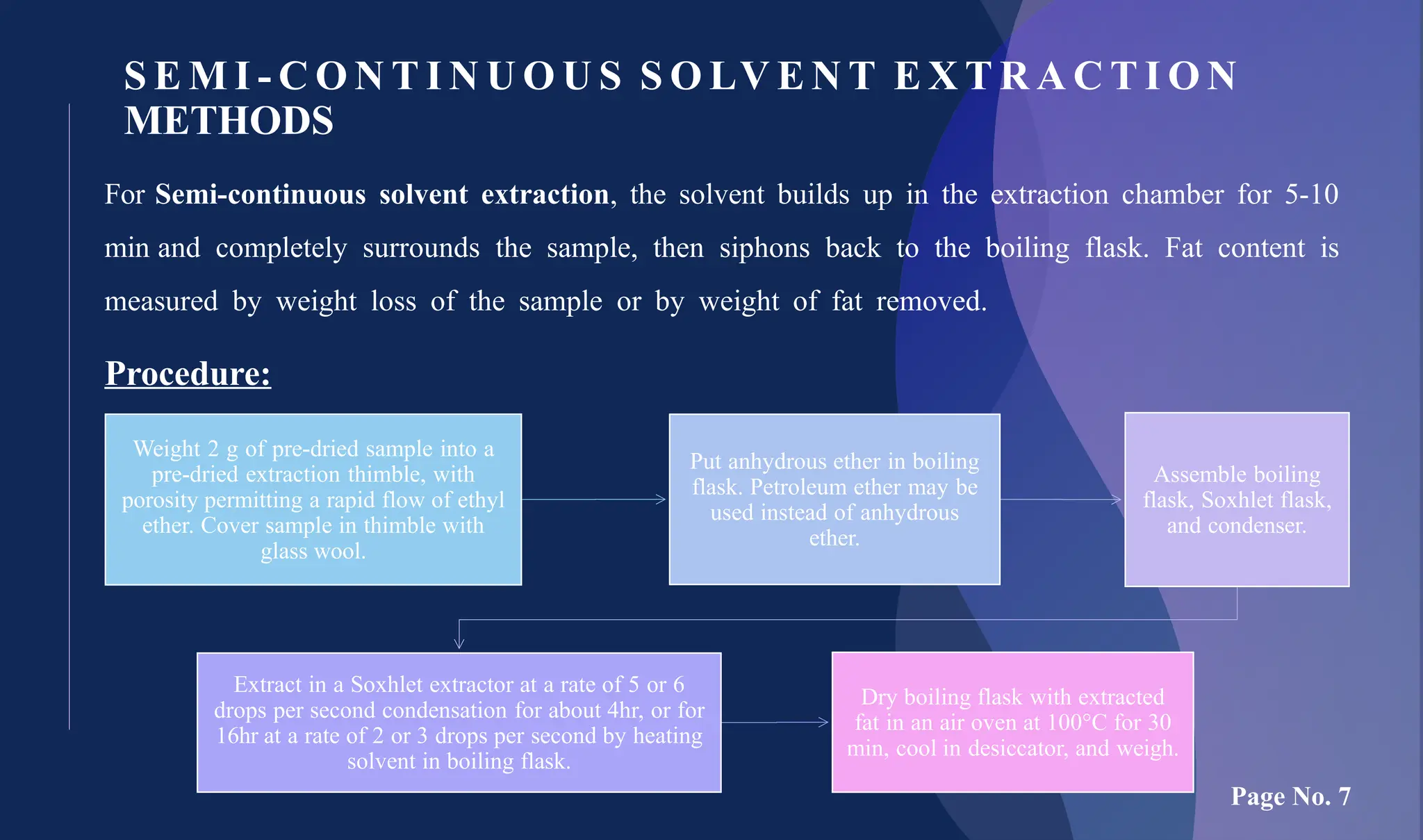
The document provides an overview of lipid analysis methods, including sample preparation and various analytical techniques such as solvent extraction, non-solvent wet extraction, and instrumental methods. Key procedures like the Mojonnier and Babcock methods for fat extraction are described, as well as the use of infrared and nuclear magnetic resonance spectroscopy for fat content determination. The conclusion emphasizes that while traditional methods like Soxhlet extraction are common, newer instrumental techniques offer quicker and more reproducible results for specific food types.