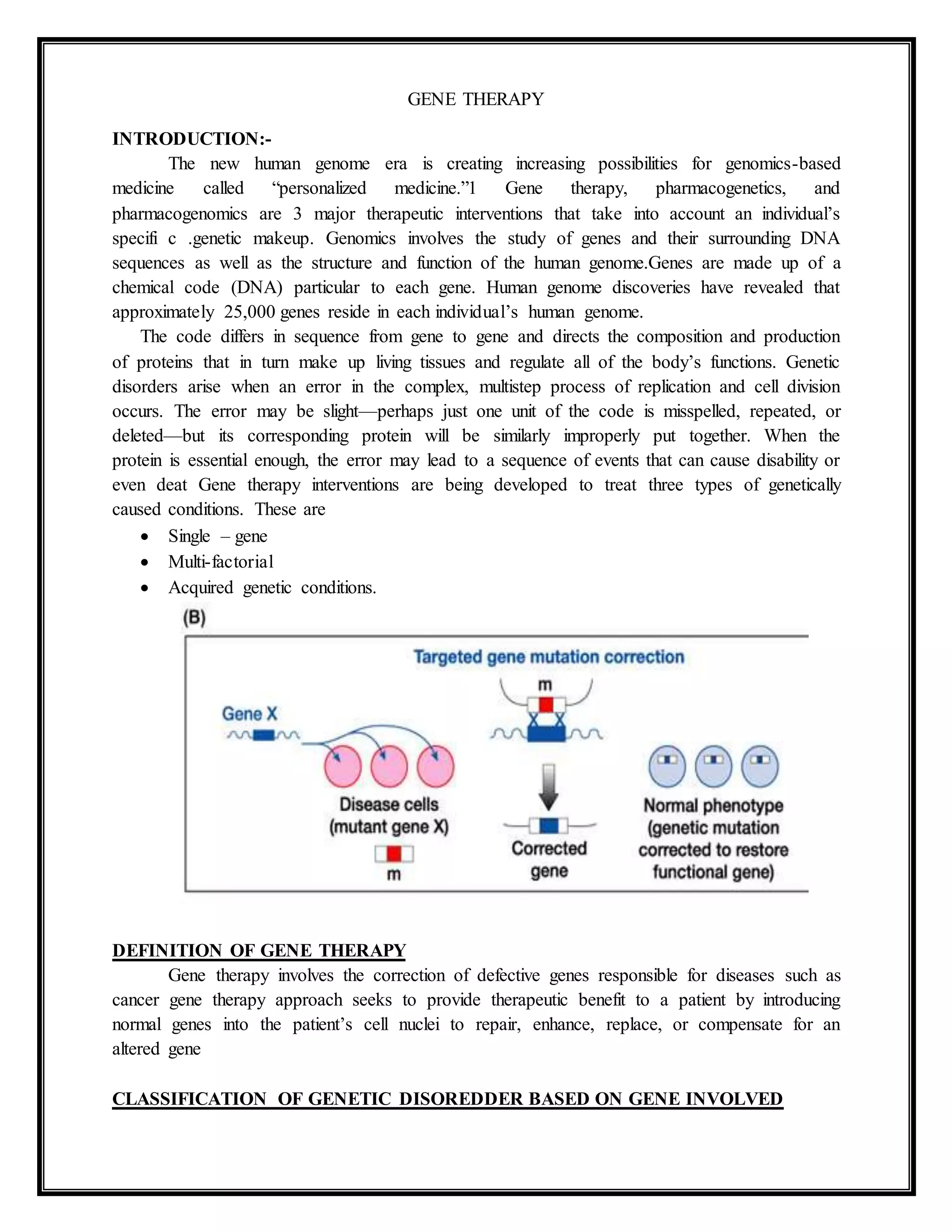
The document provides an overview of gene therapy, highlighting its potential in treating genetic disorders and cancers through the correction of defective genes. It discusses various types of genetic disorders, gene therapy principles, methods of gene transfer, and the challenges associated with different viral vectors used in the process. Additionally, it outlines the clinical protocols, regulation of gene therapy, and explores the concept of cancer gene therapy, including approaches like oncolytic viral therapy and immunotherapy.