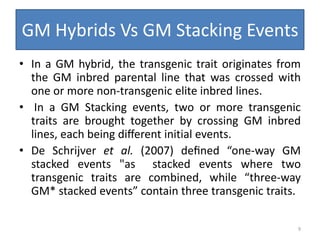
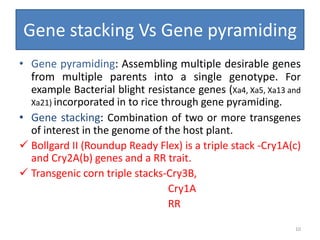
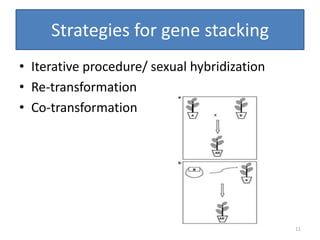
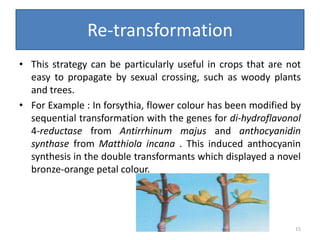
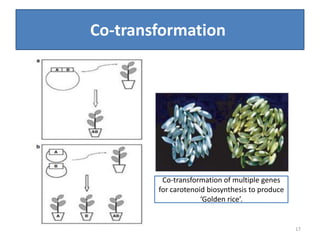
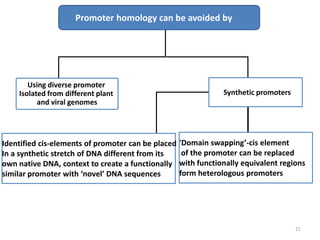
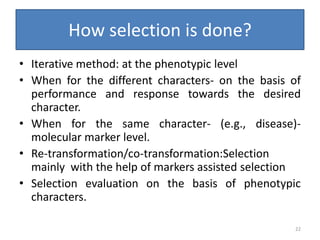
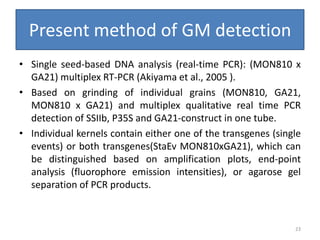
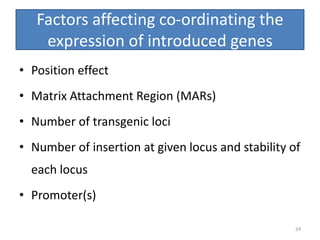
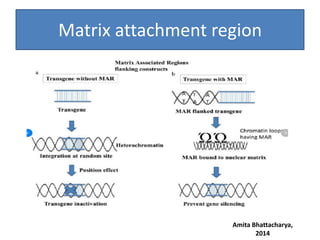
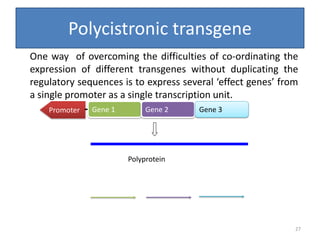
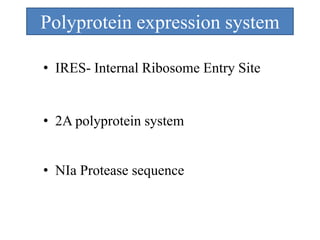
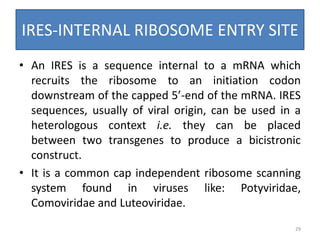
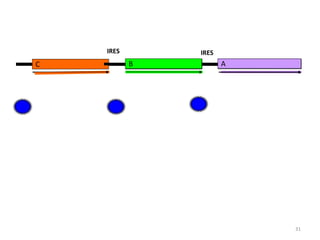
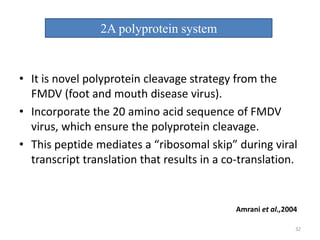
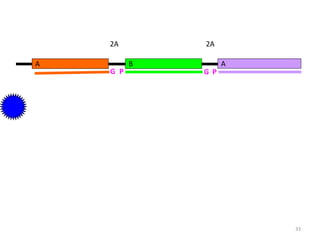
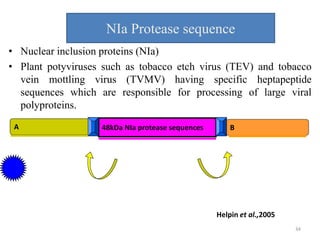
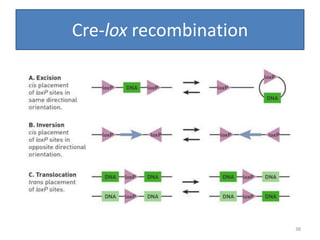
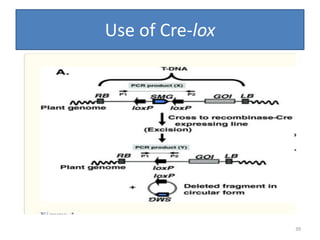
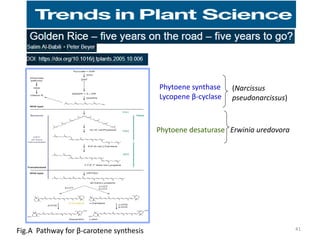
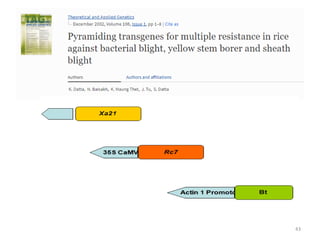
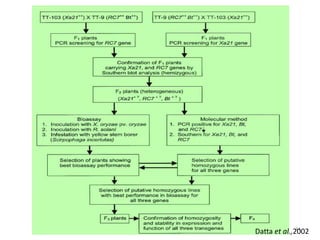
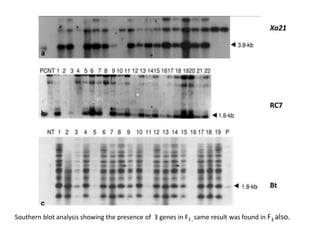
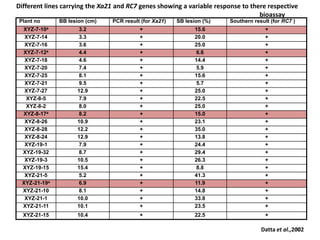
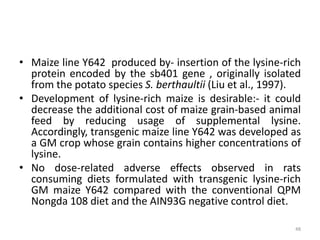
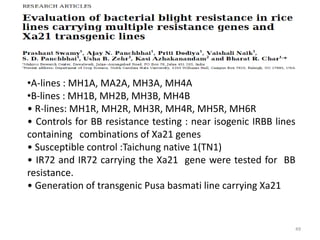
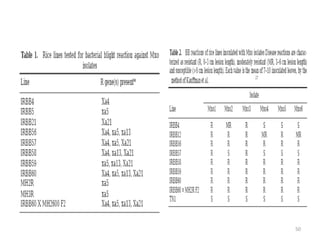
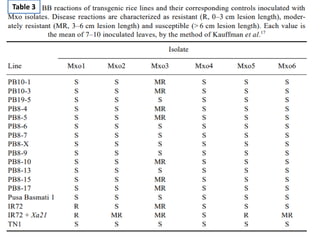
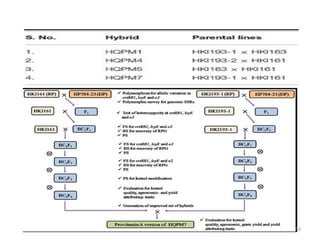
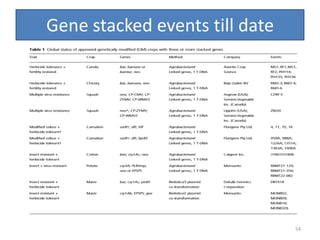
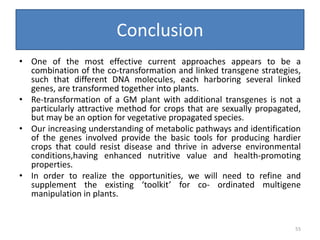
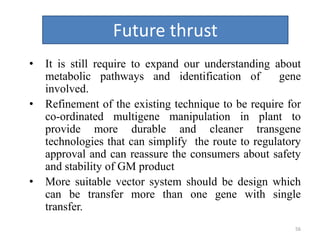
Gene stacking involves combining two or more transgenes into a host plant genome. It can be achieved through iterative crossing of transgenic plants, re-transformation of transgenic plants with additional genes, or co-transformation of multiple genes simultaneously. Co-transformation allows multiple genes to be introduced together but risks silencing effects if the same promoter is used. Iterative crossing is time-consuming but avoids this issue. Gene stacking holds promise for improving crop traits like disease resistance and nutrition but careful selection is needed to maintain expression levels of all genes. Recent examples demonstrate progress in stacking drought tolerance, yield, and nutrition genes into elite crop varieties.