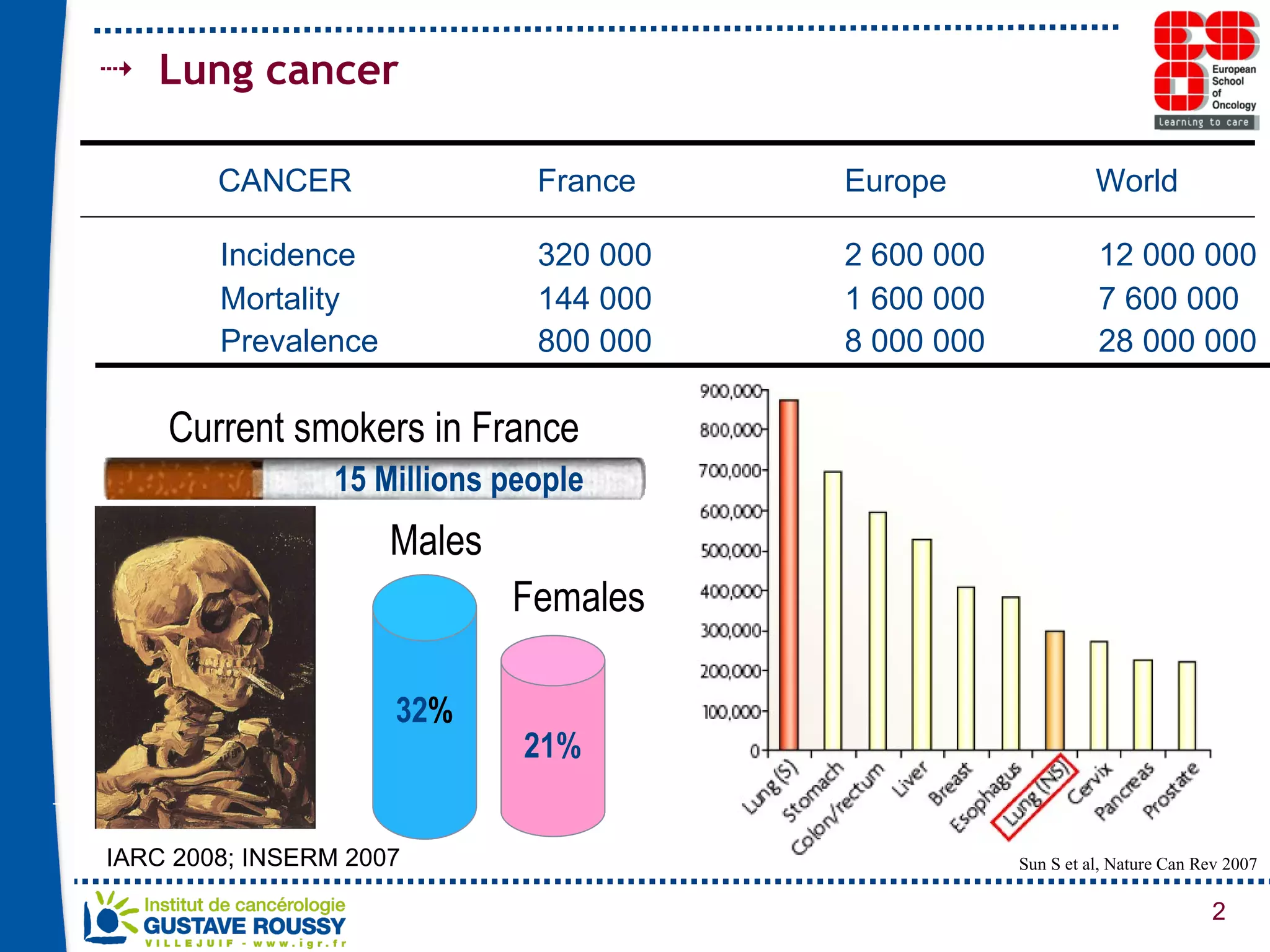
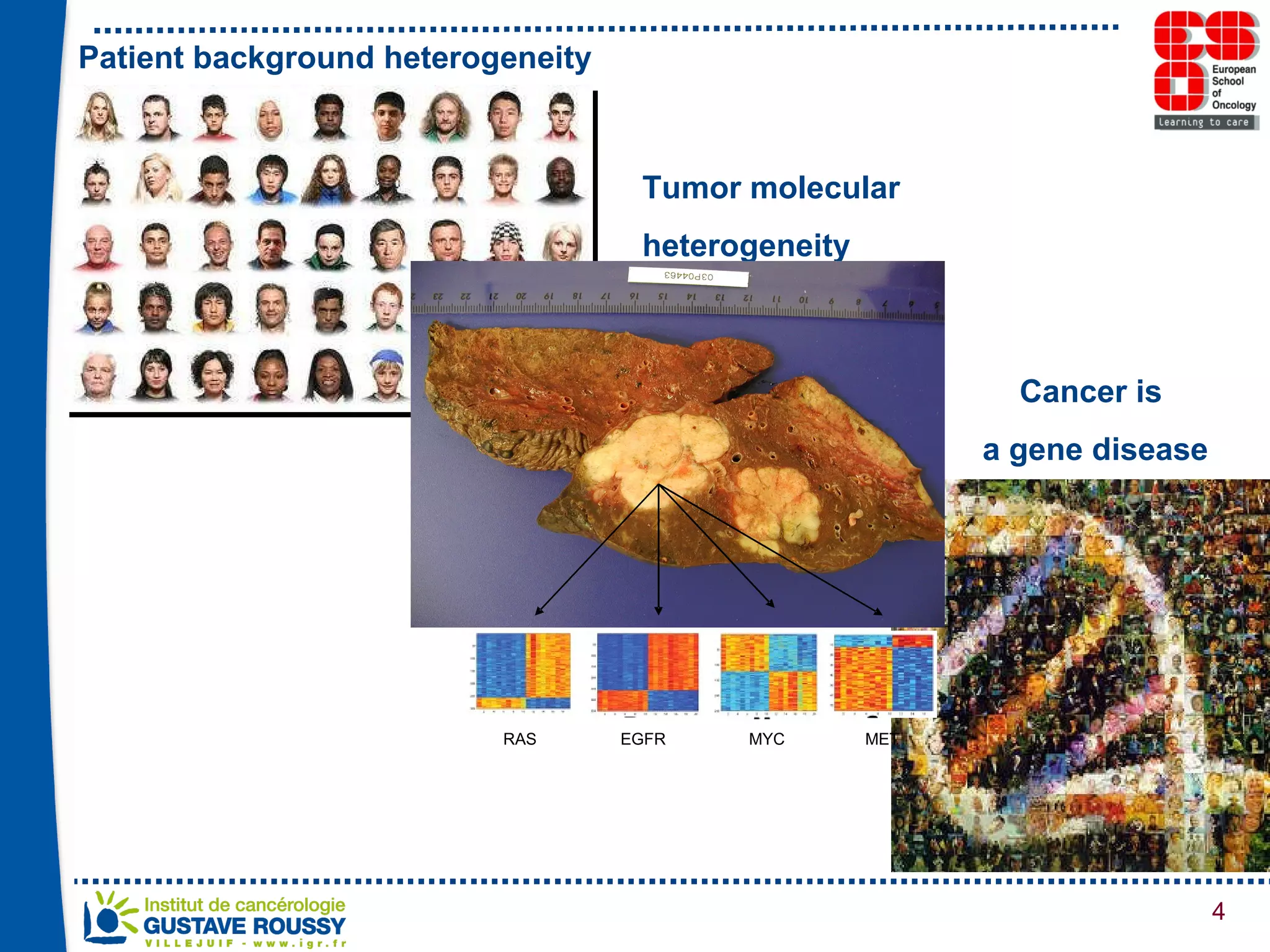
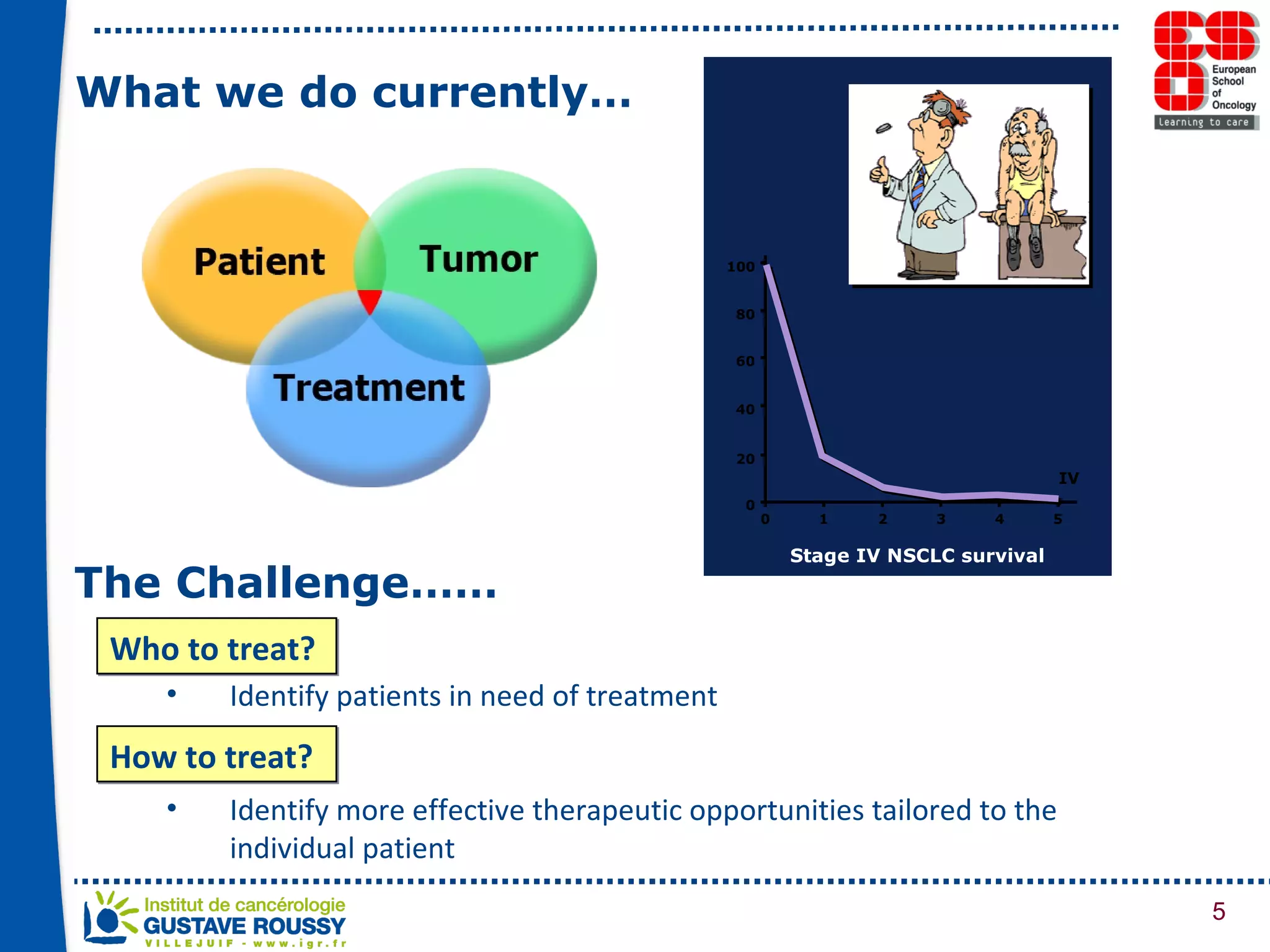
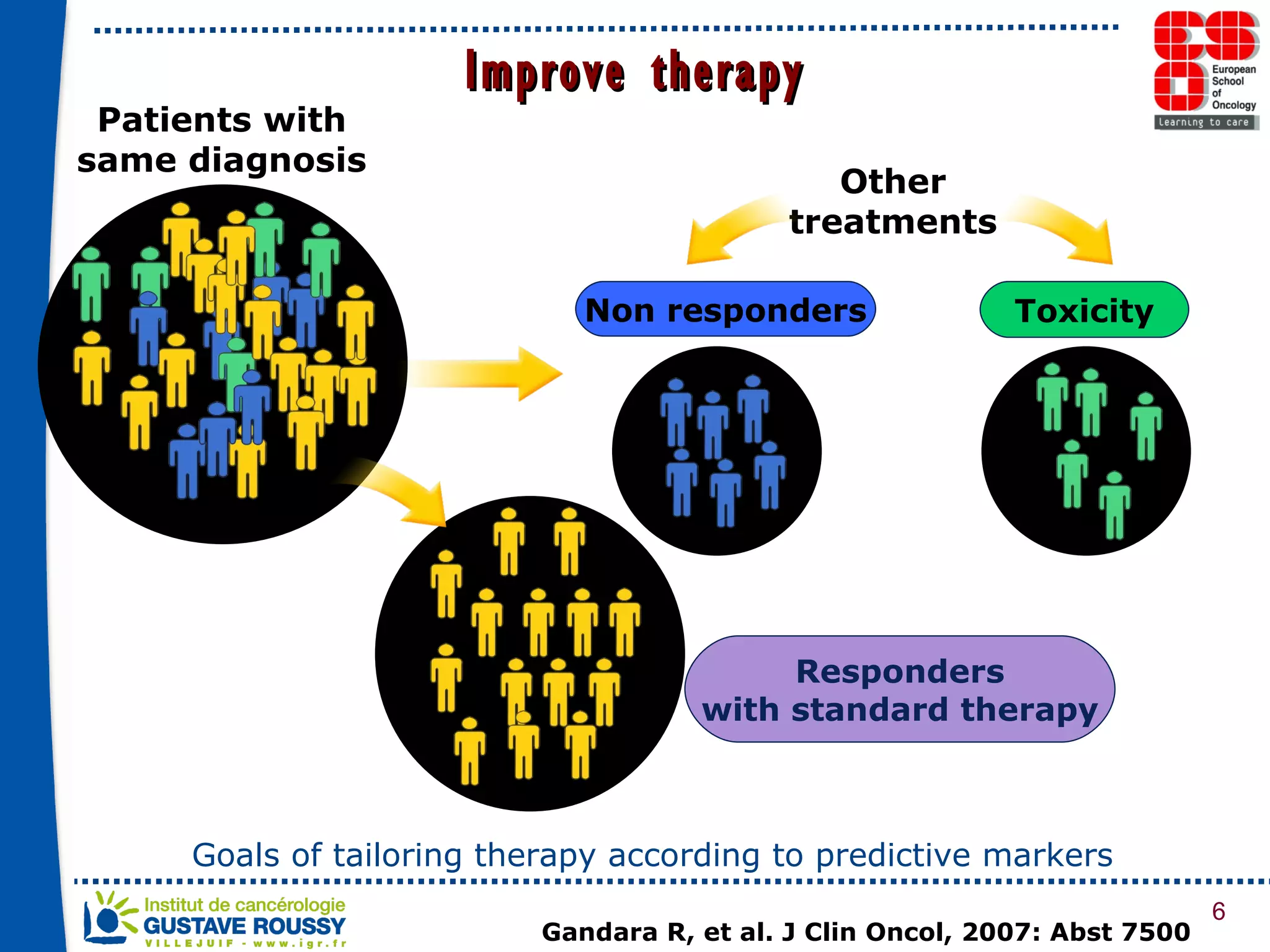
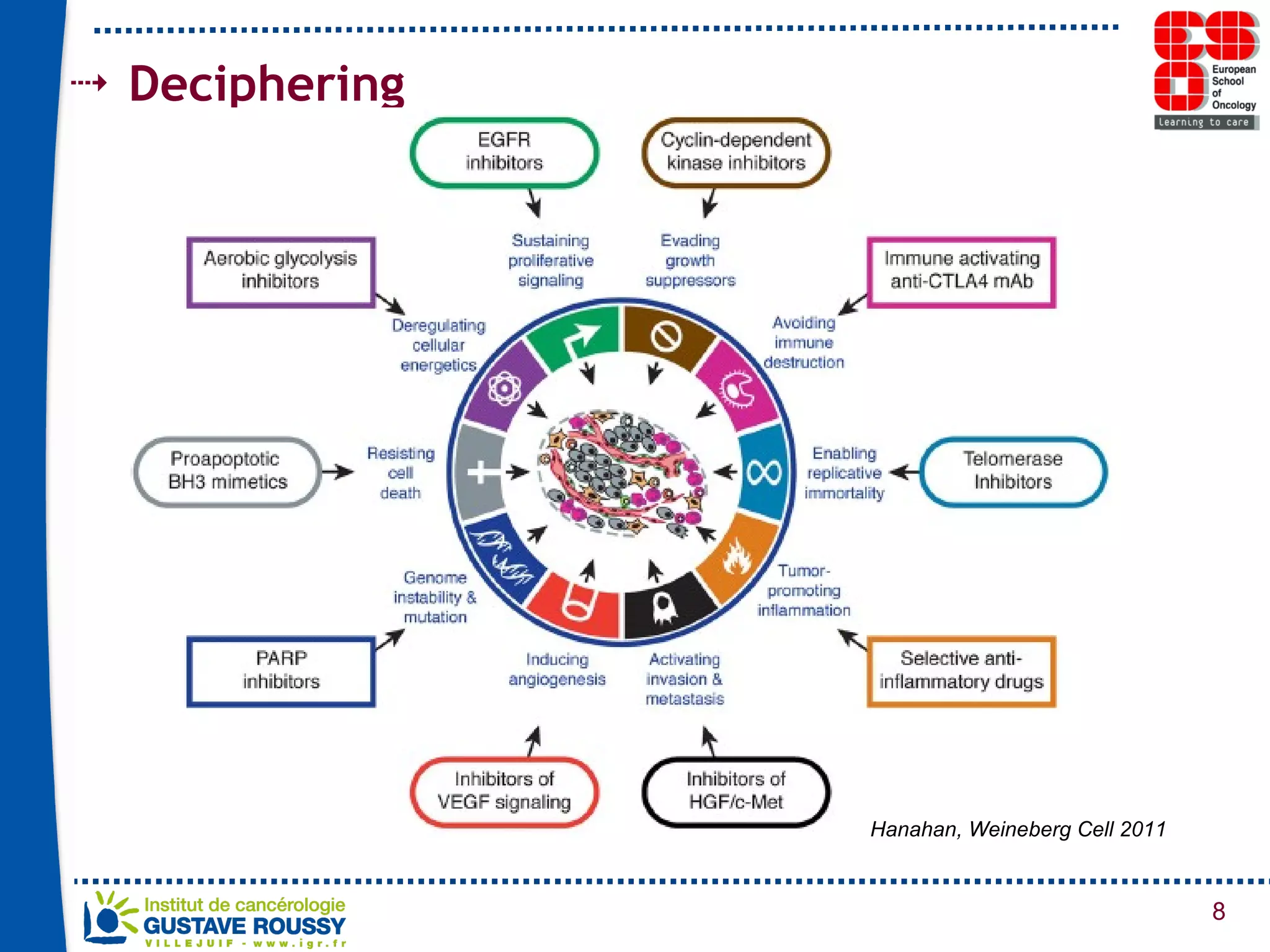
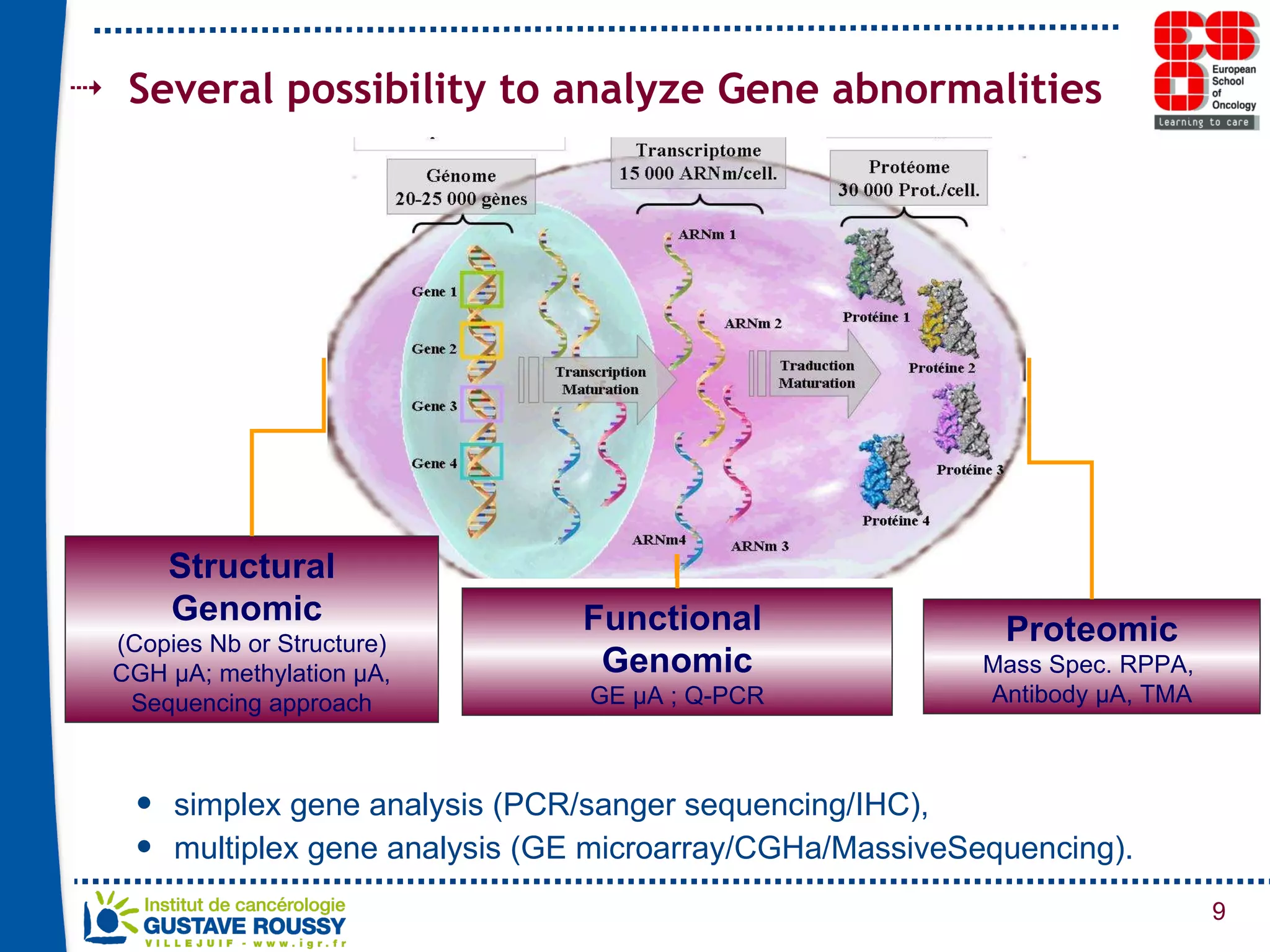
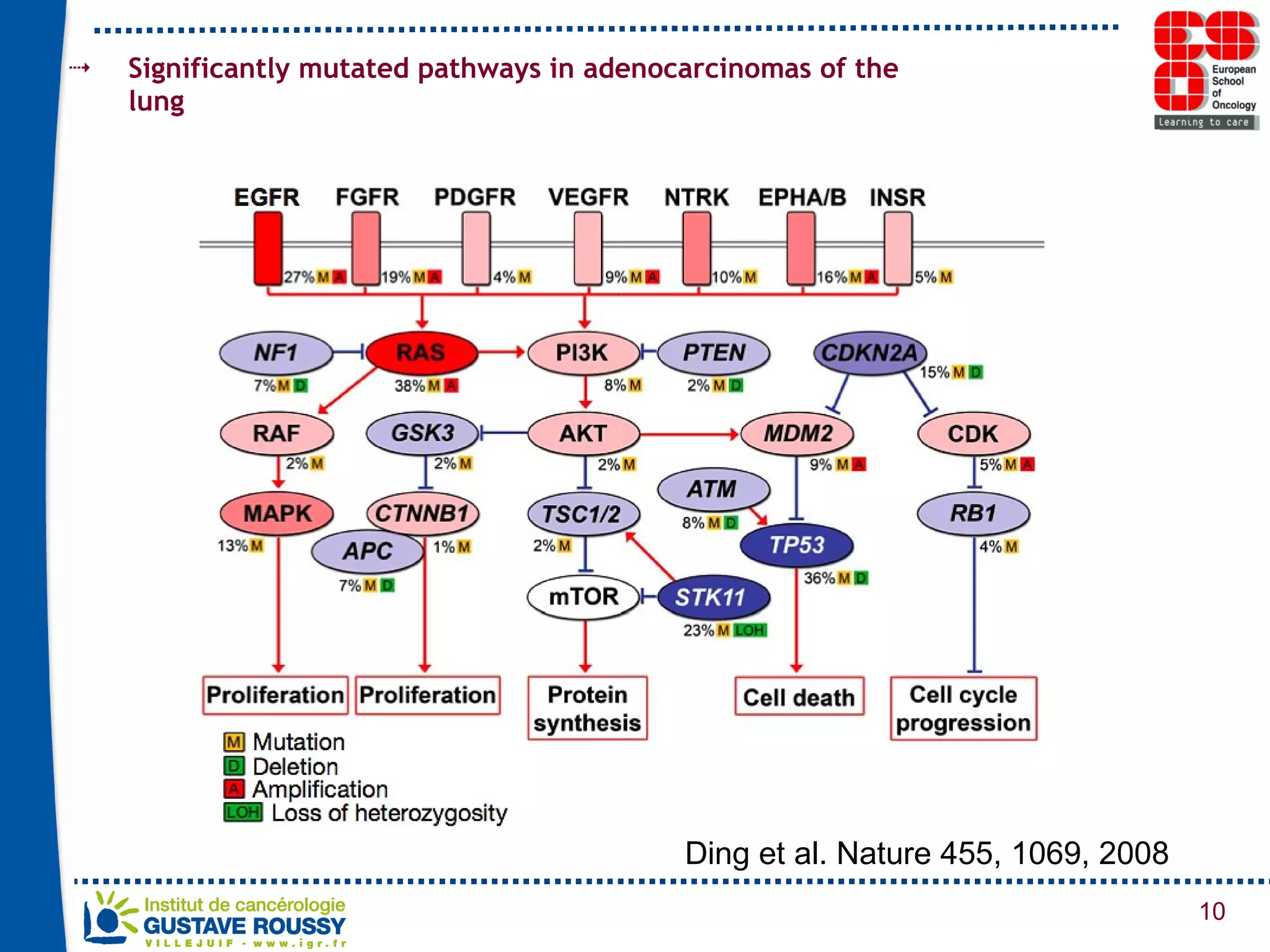
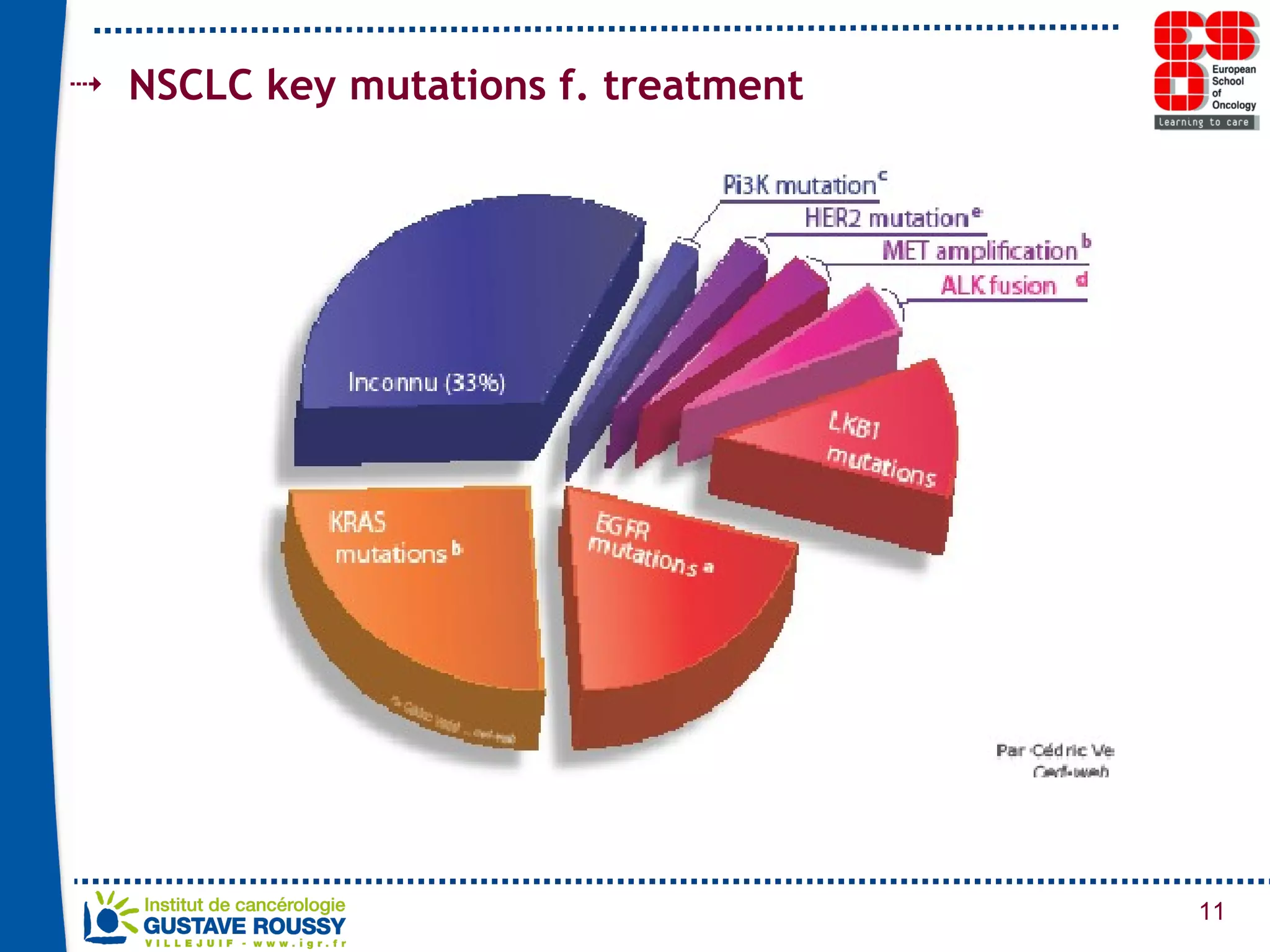
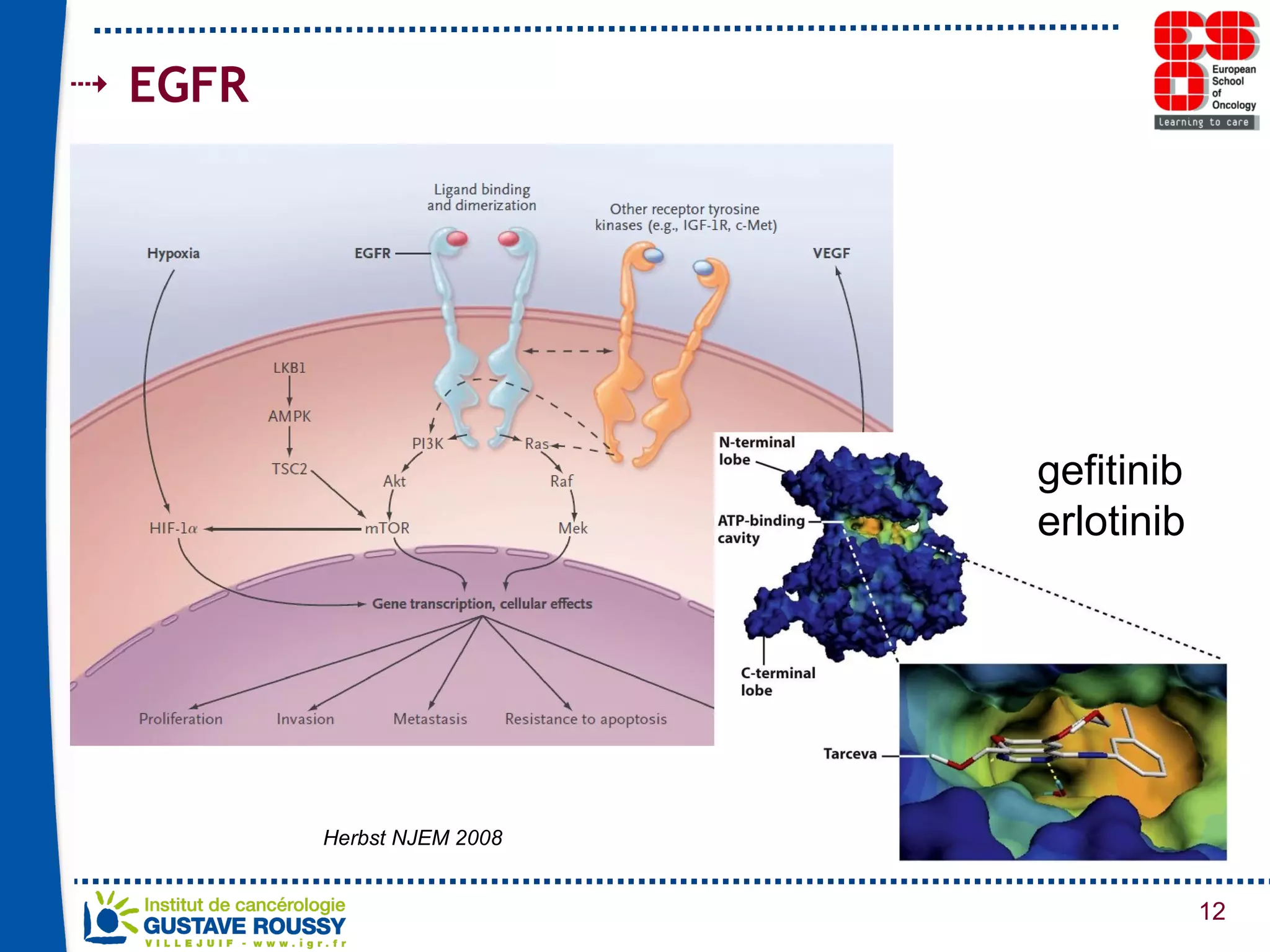
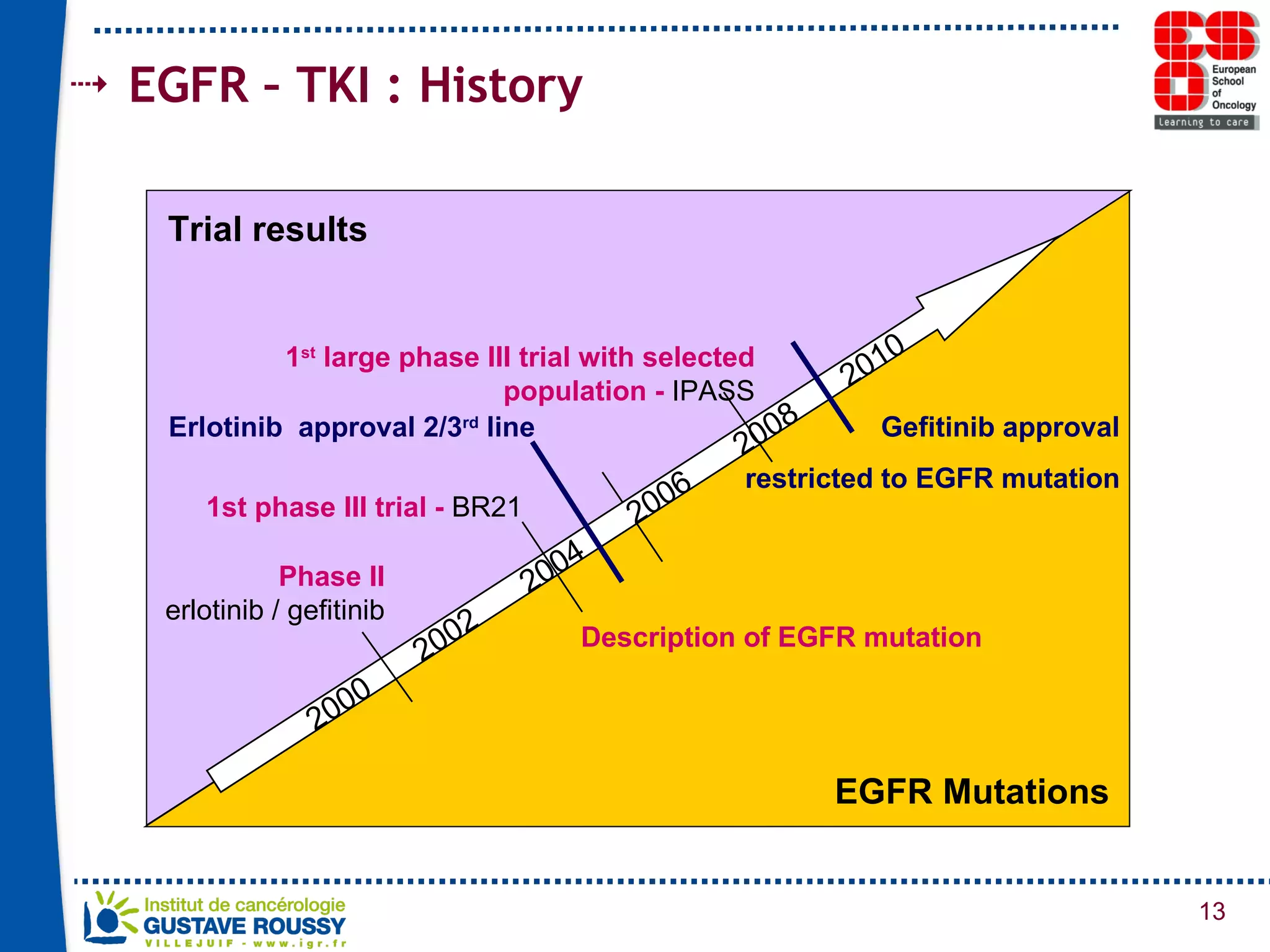
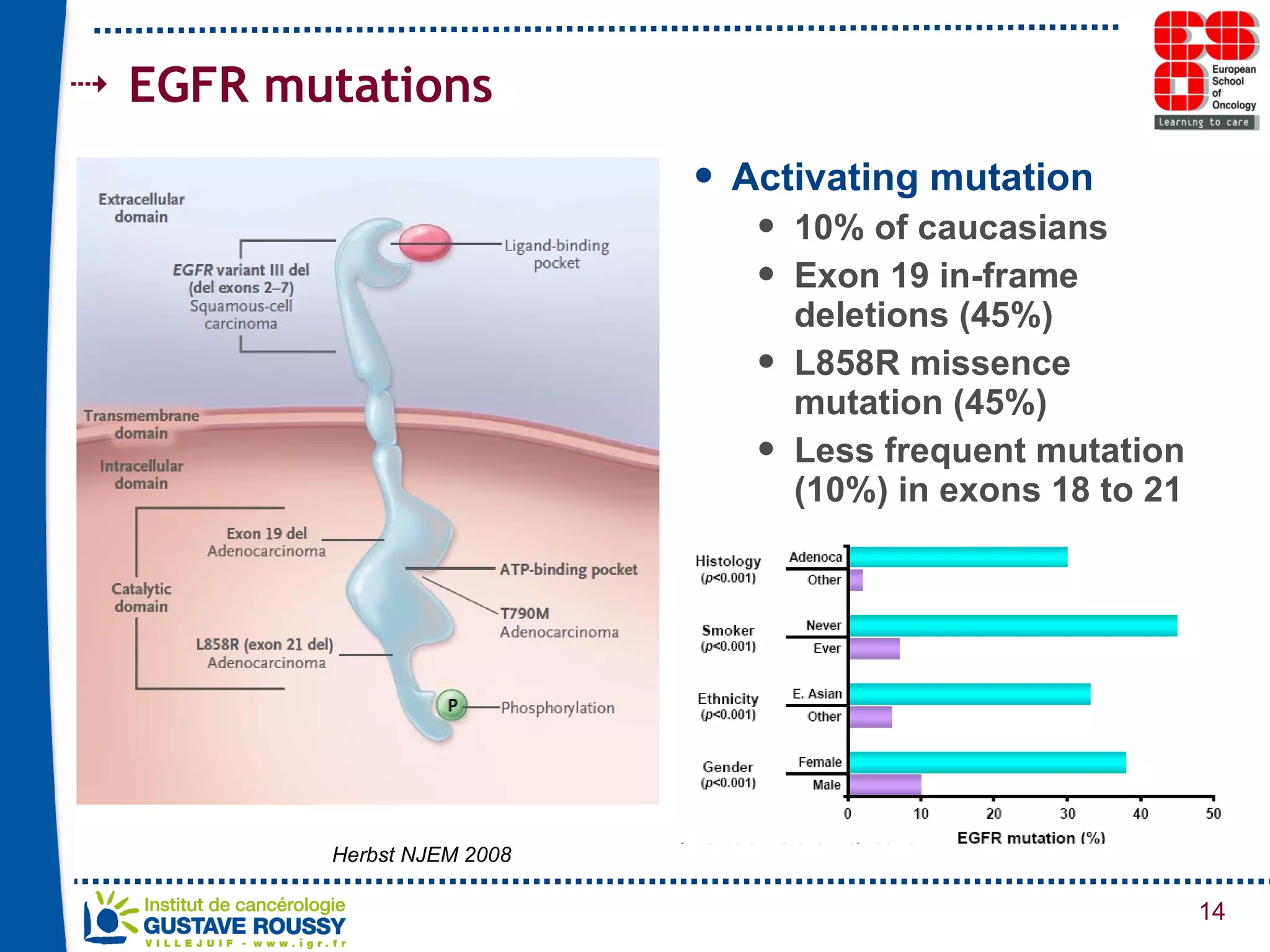
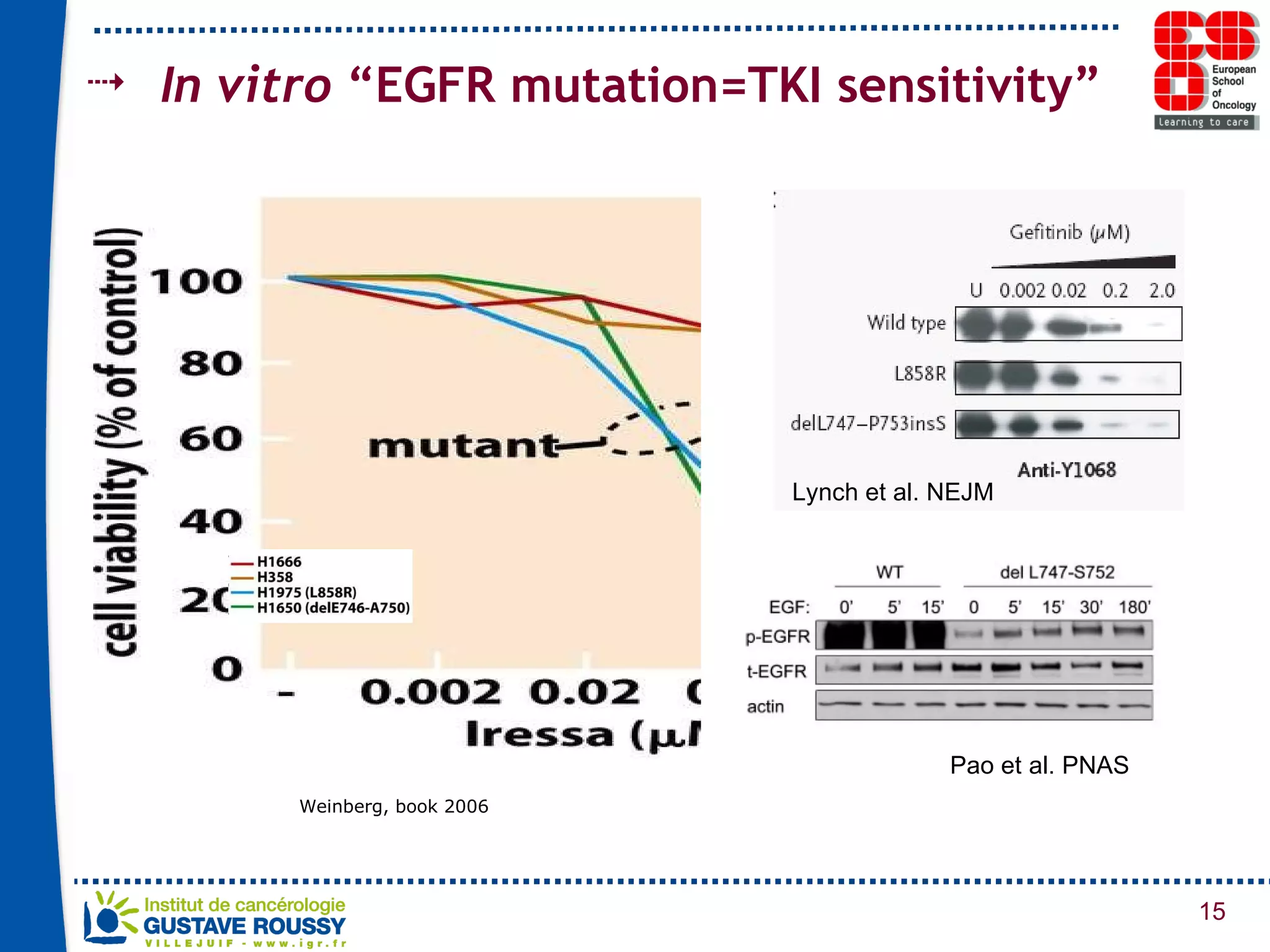
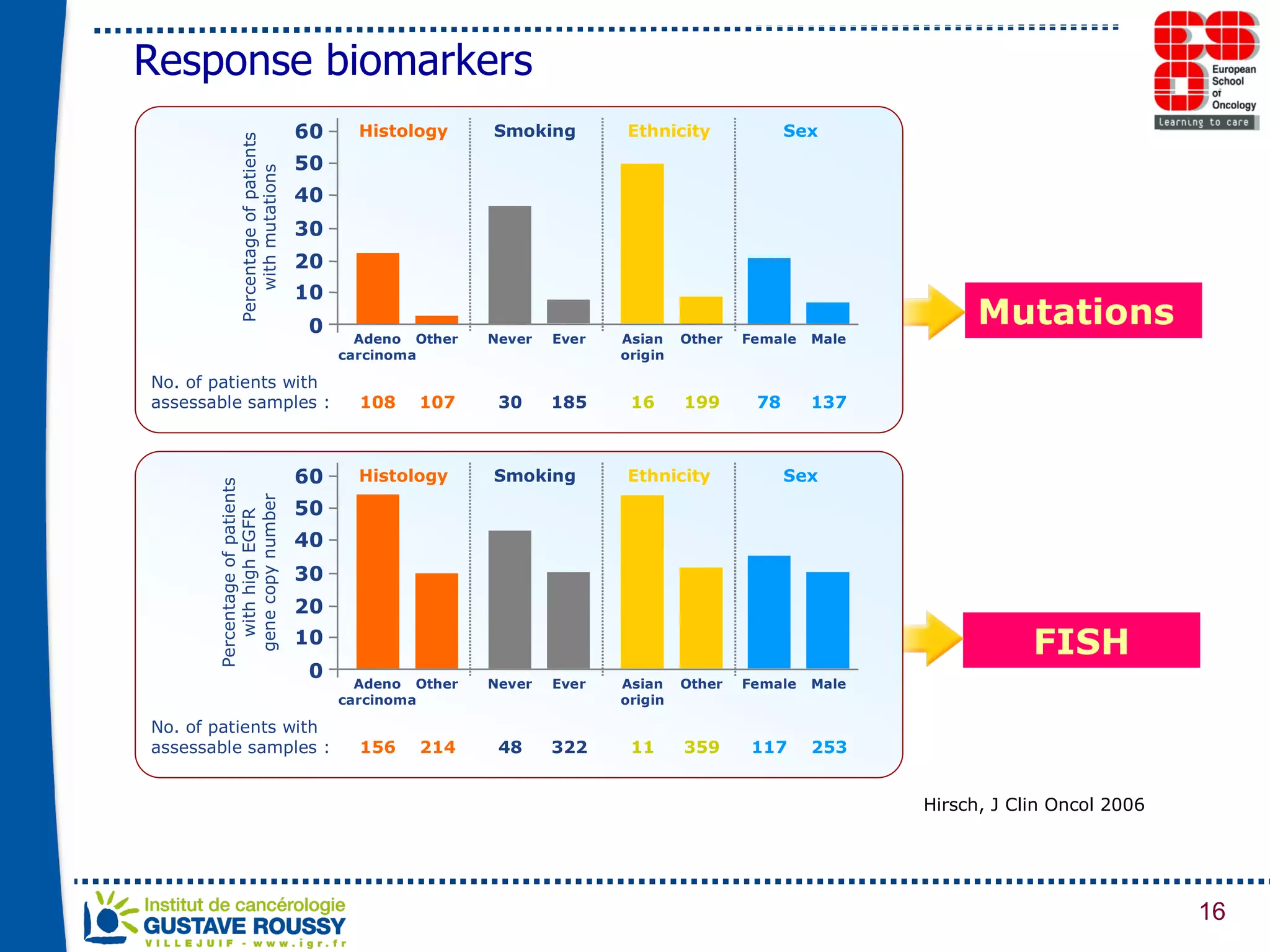
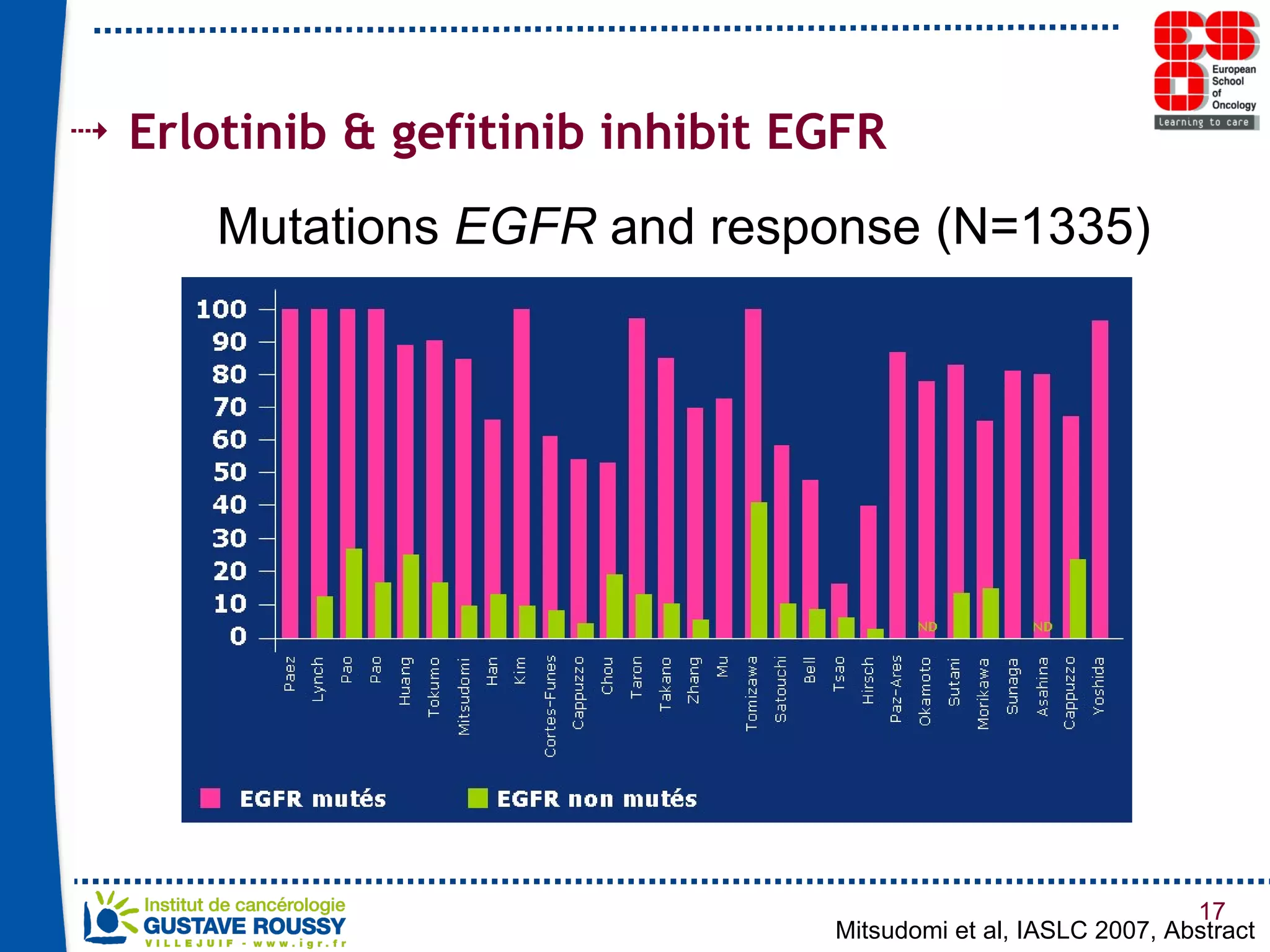
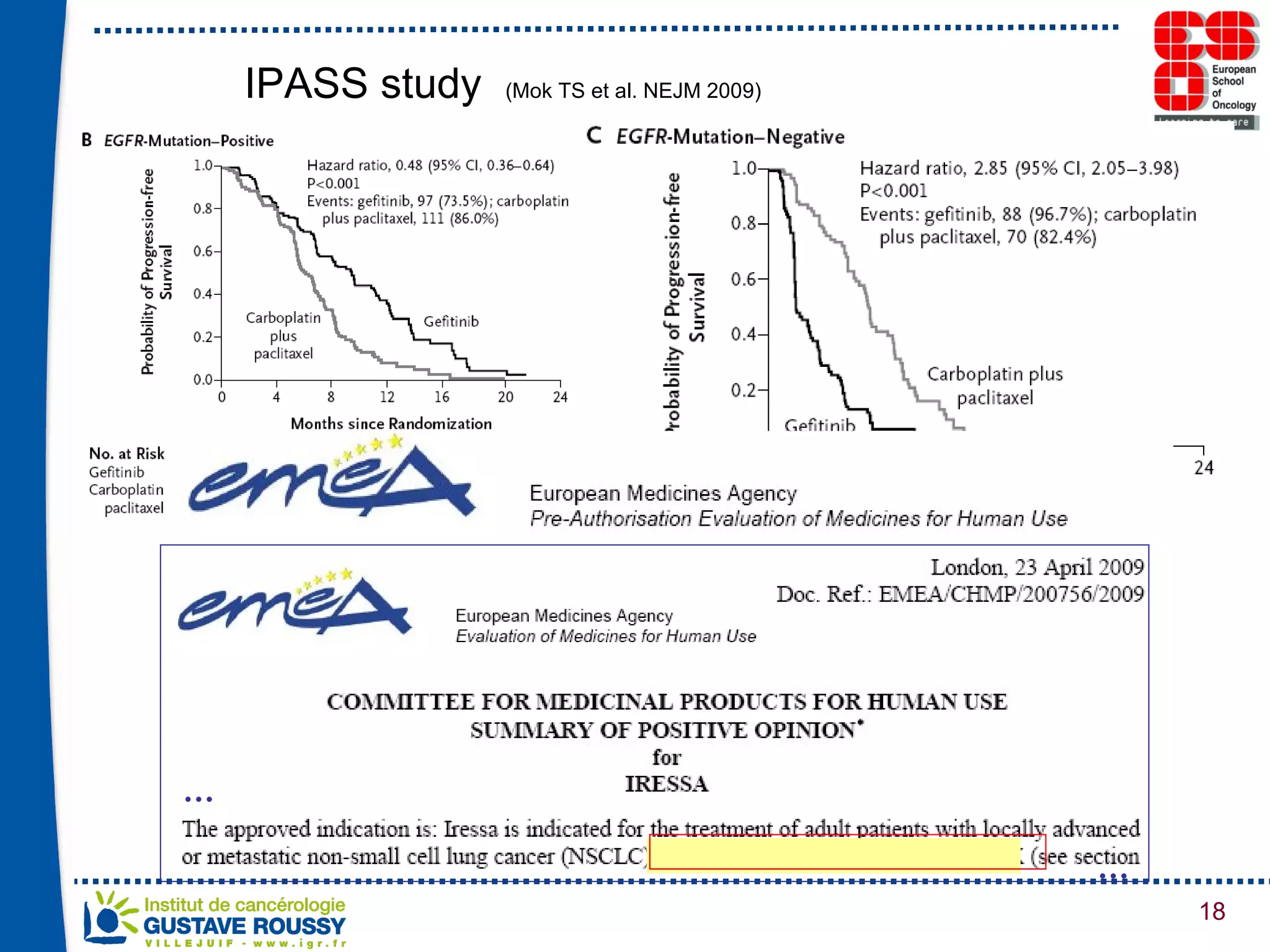
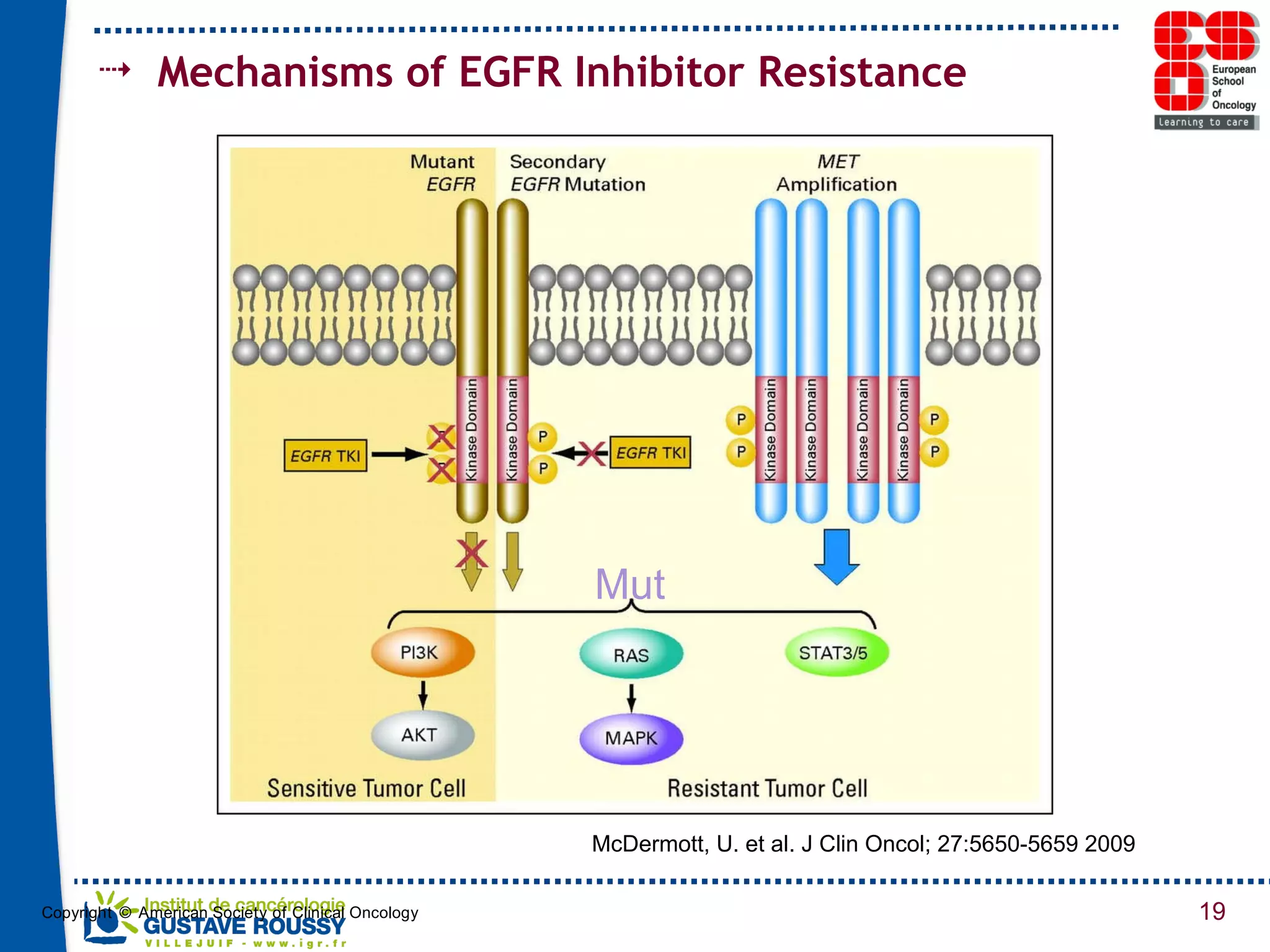
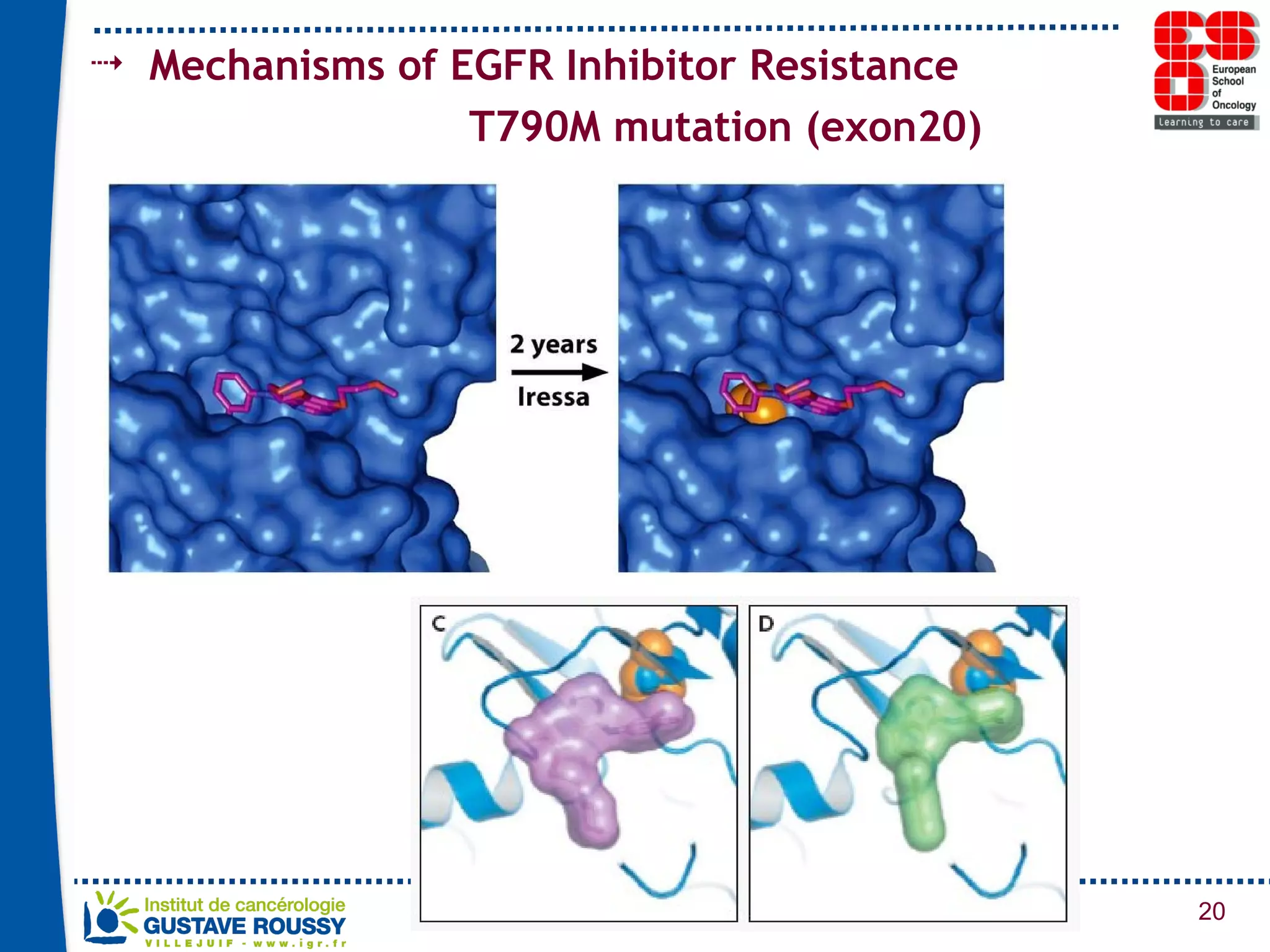
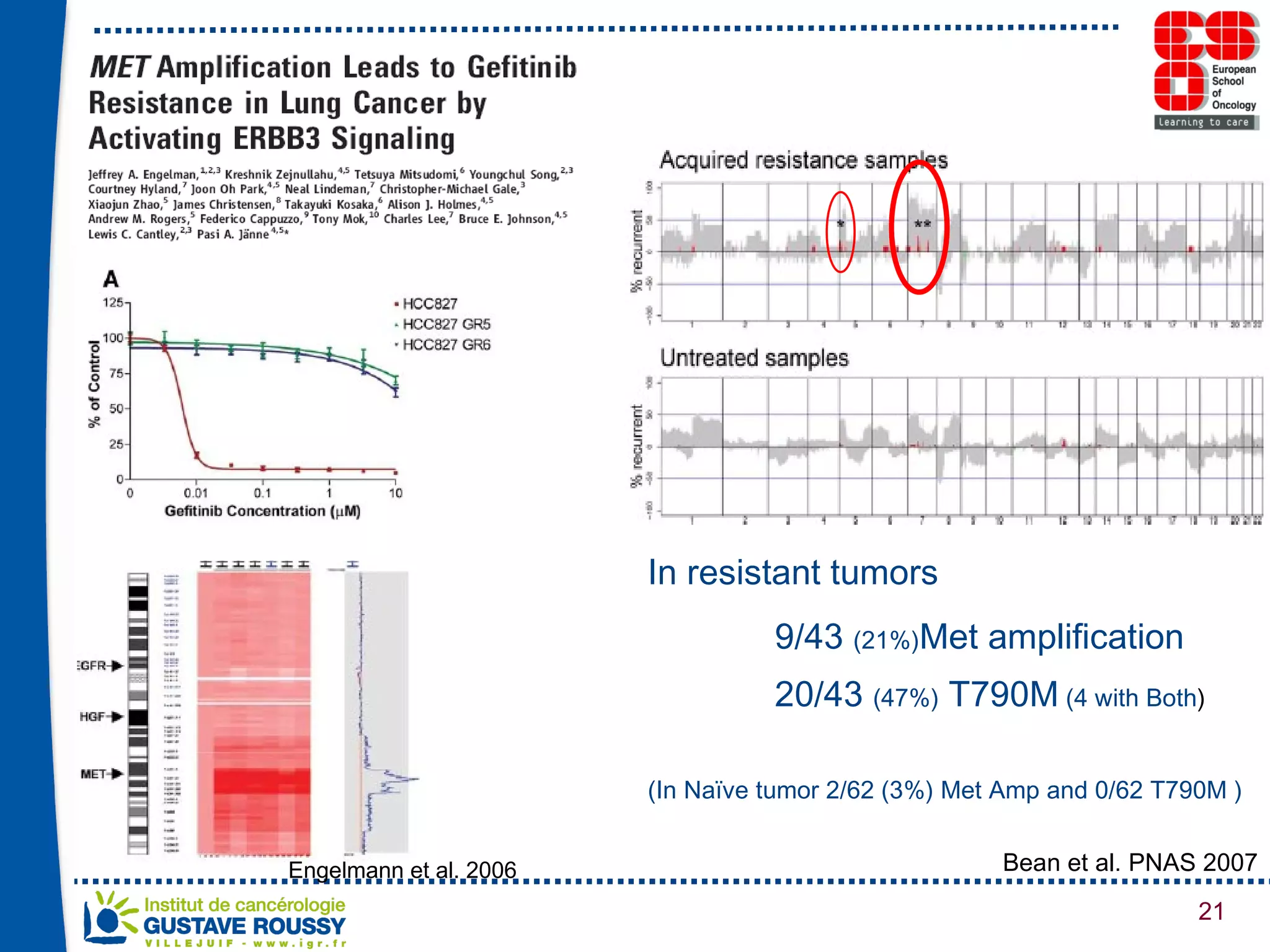
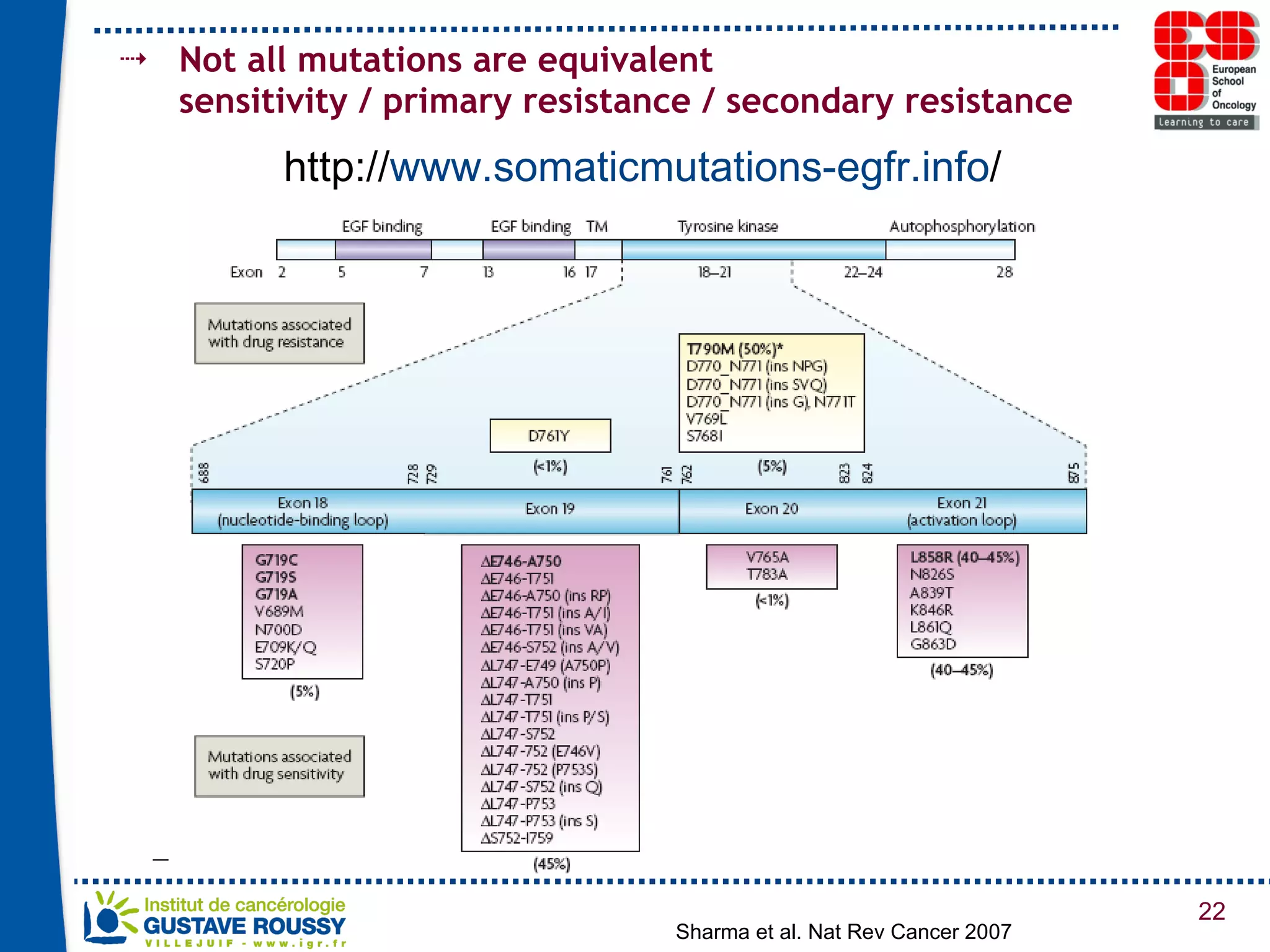
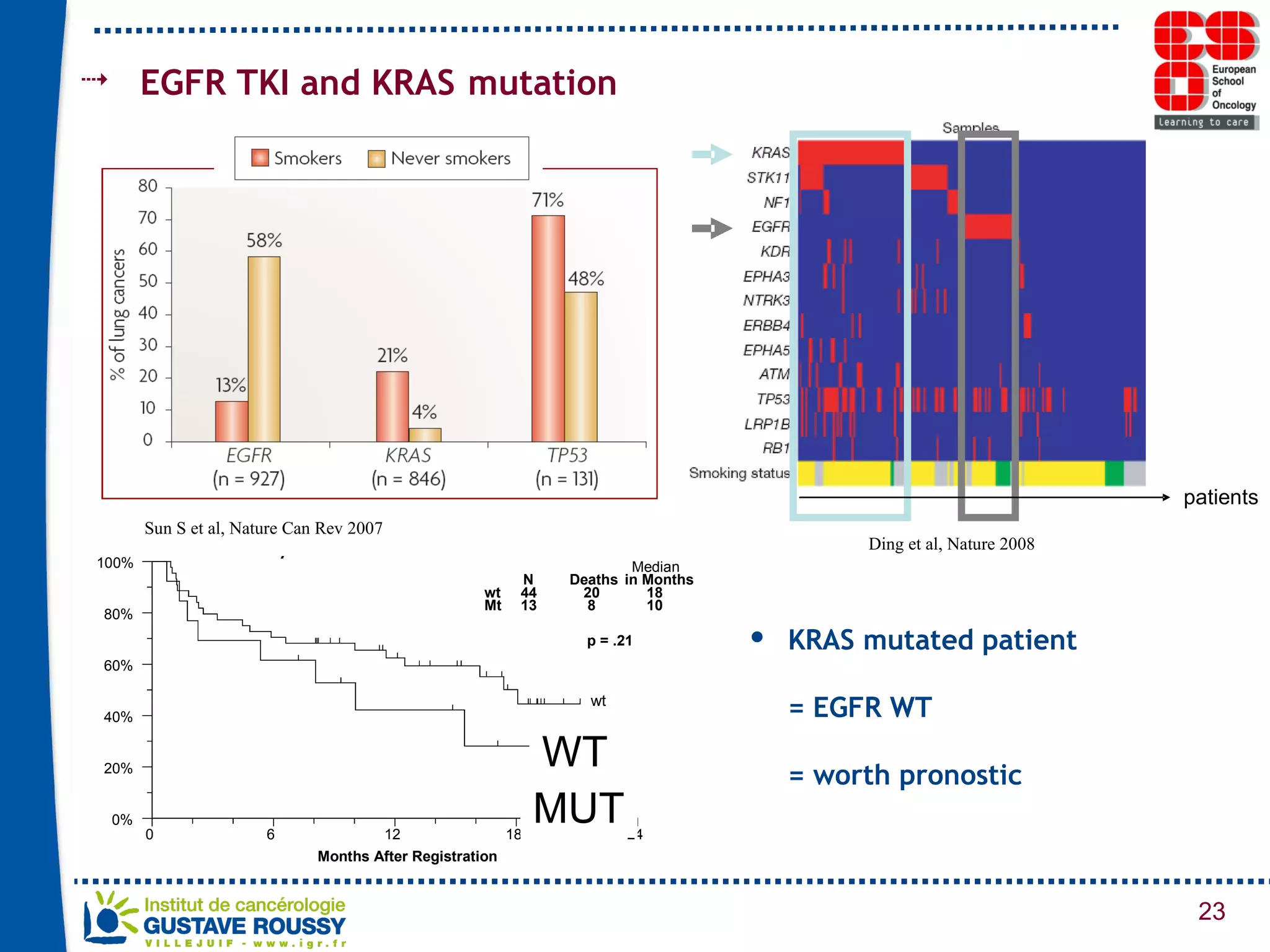
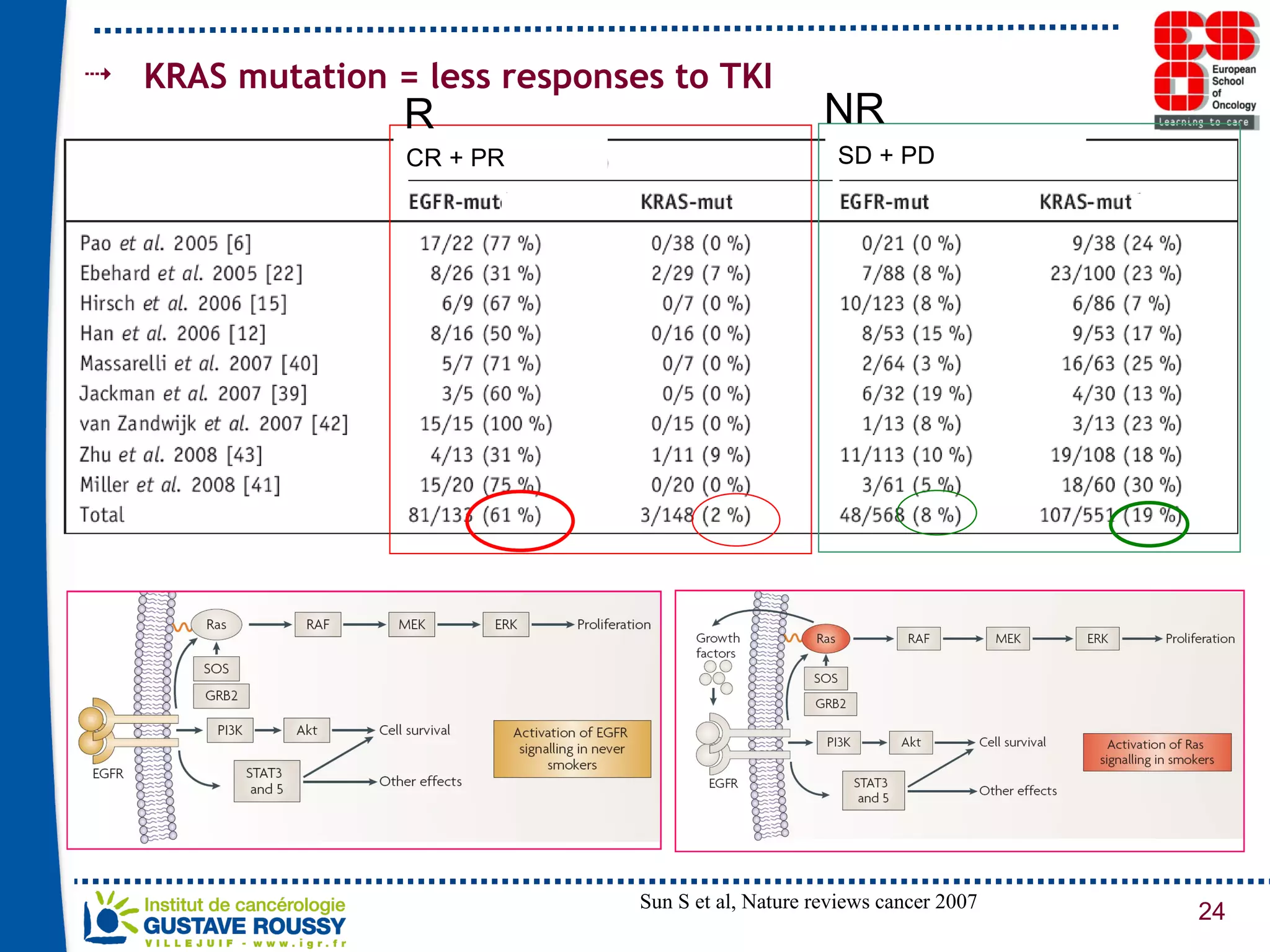
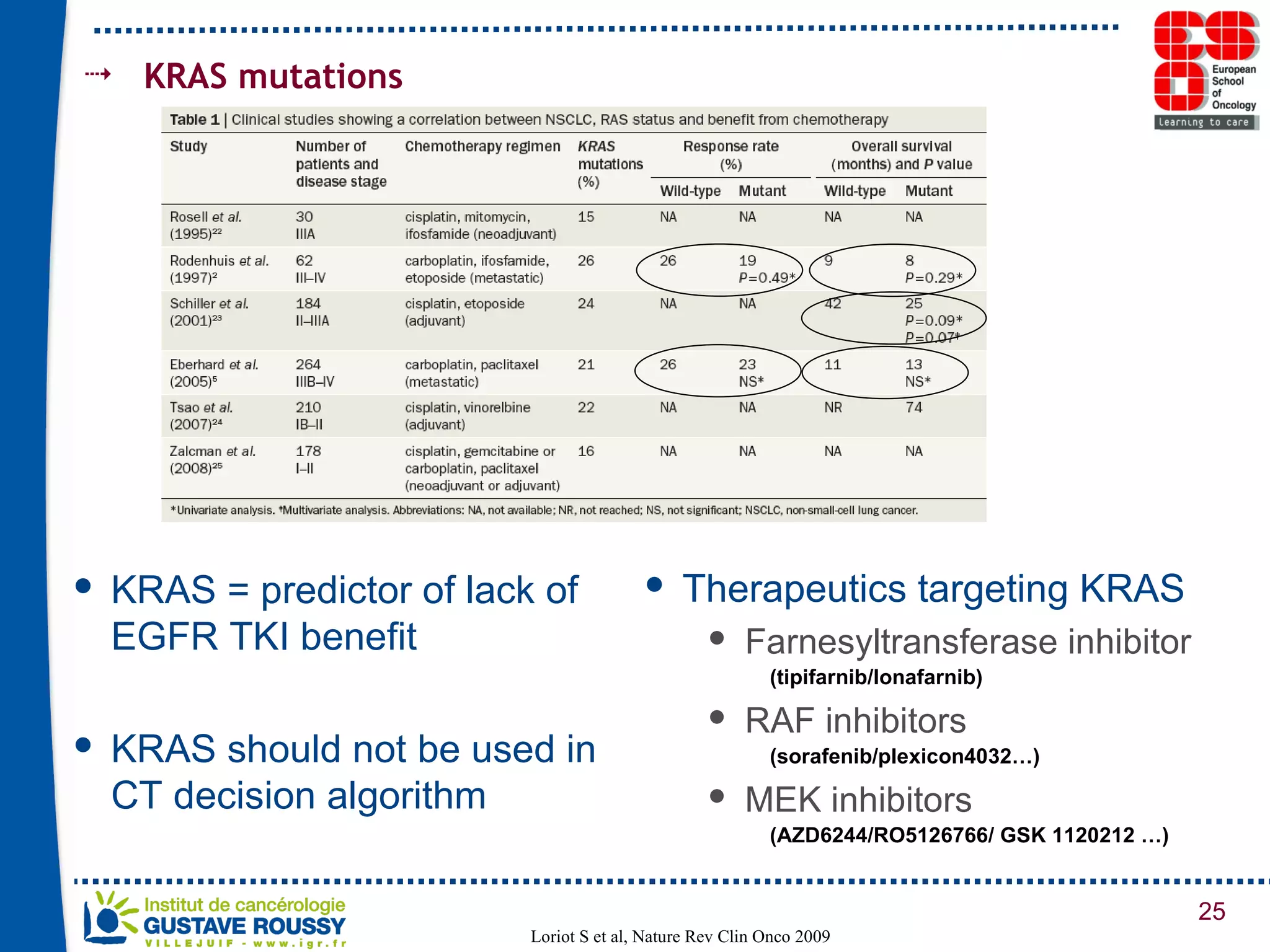
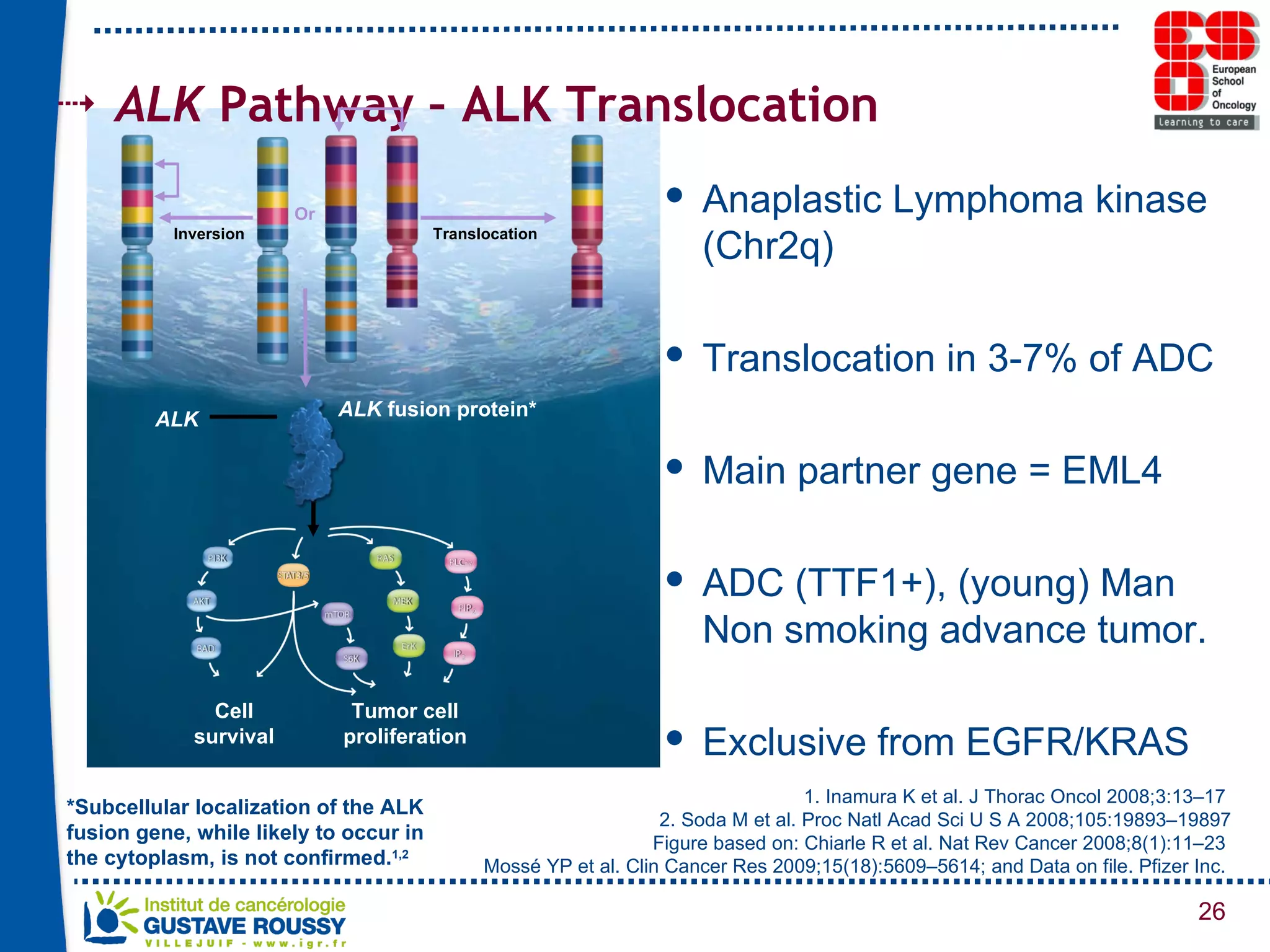
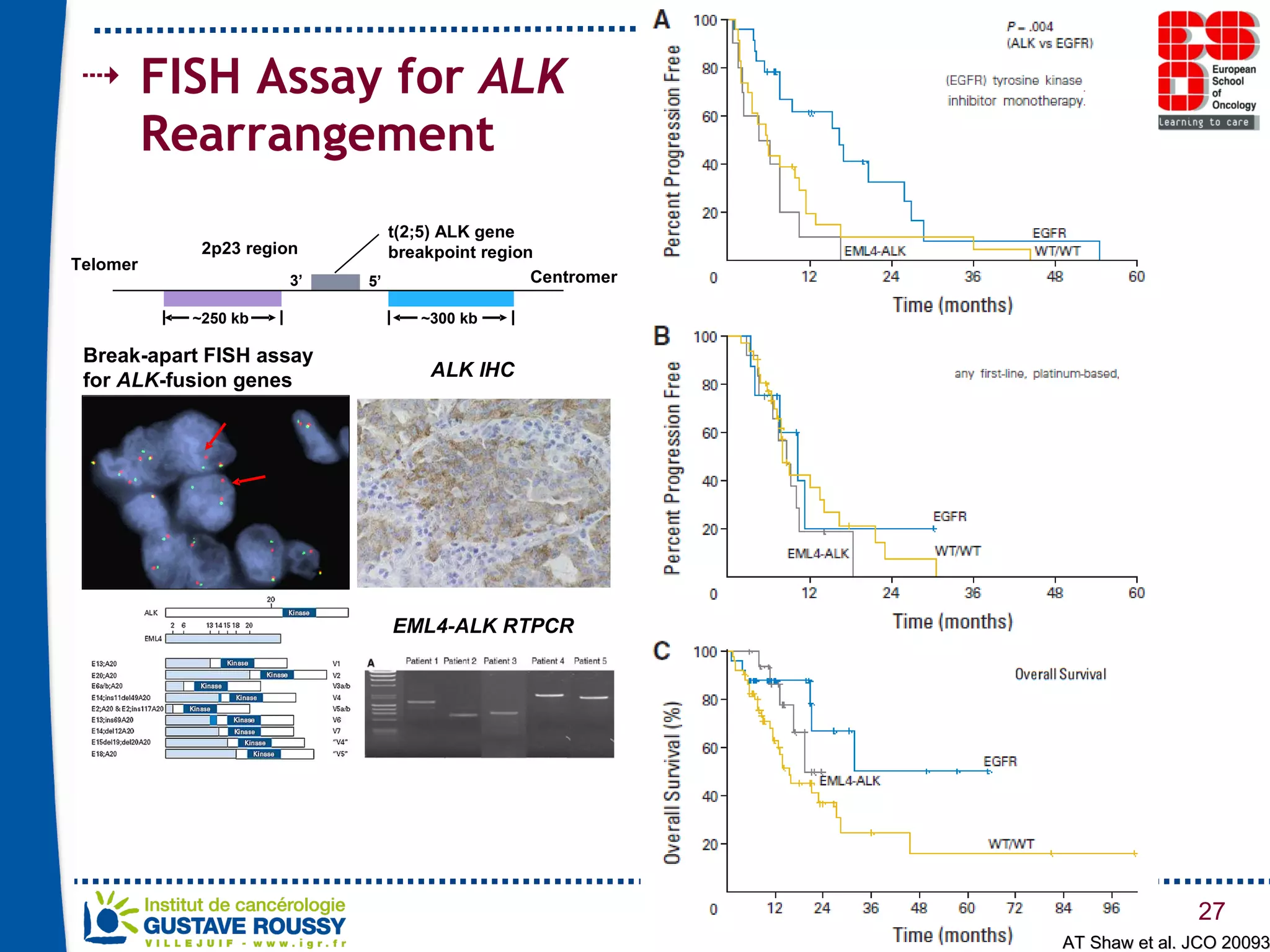
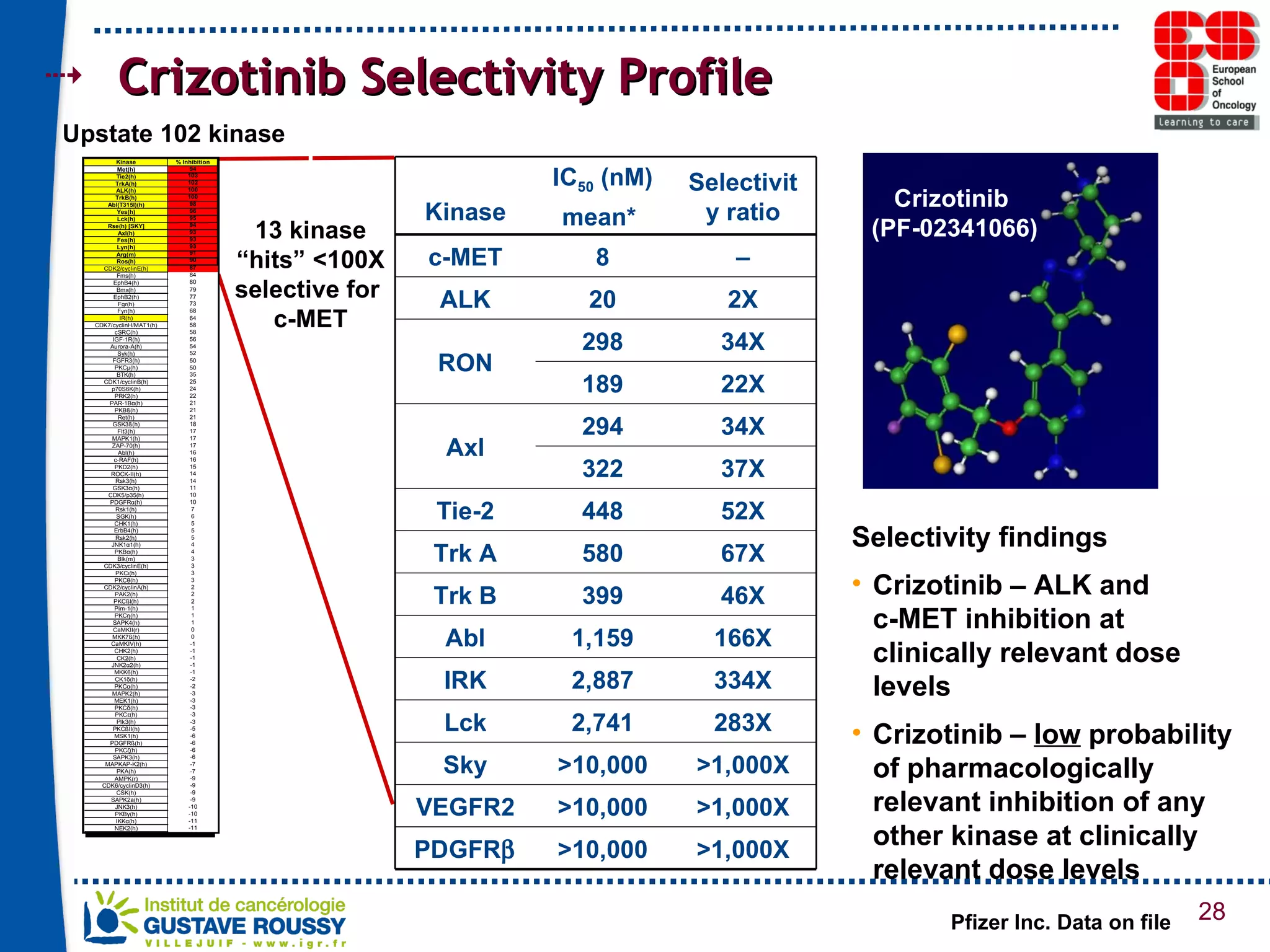
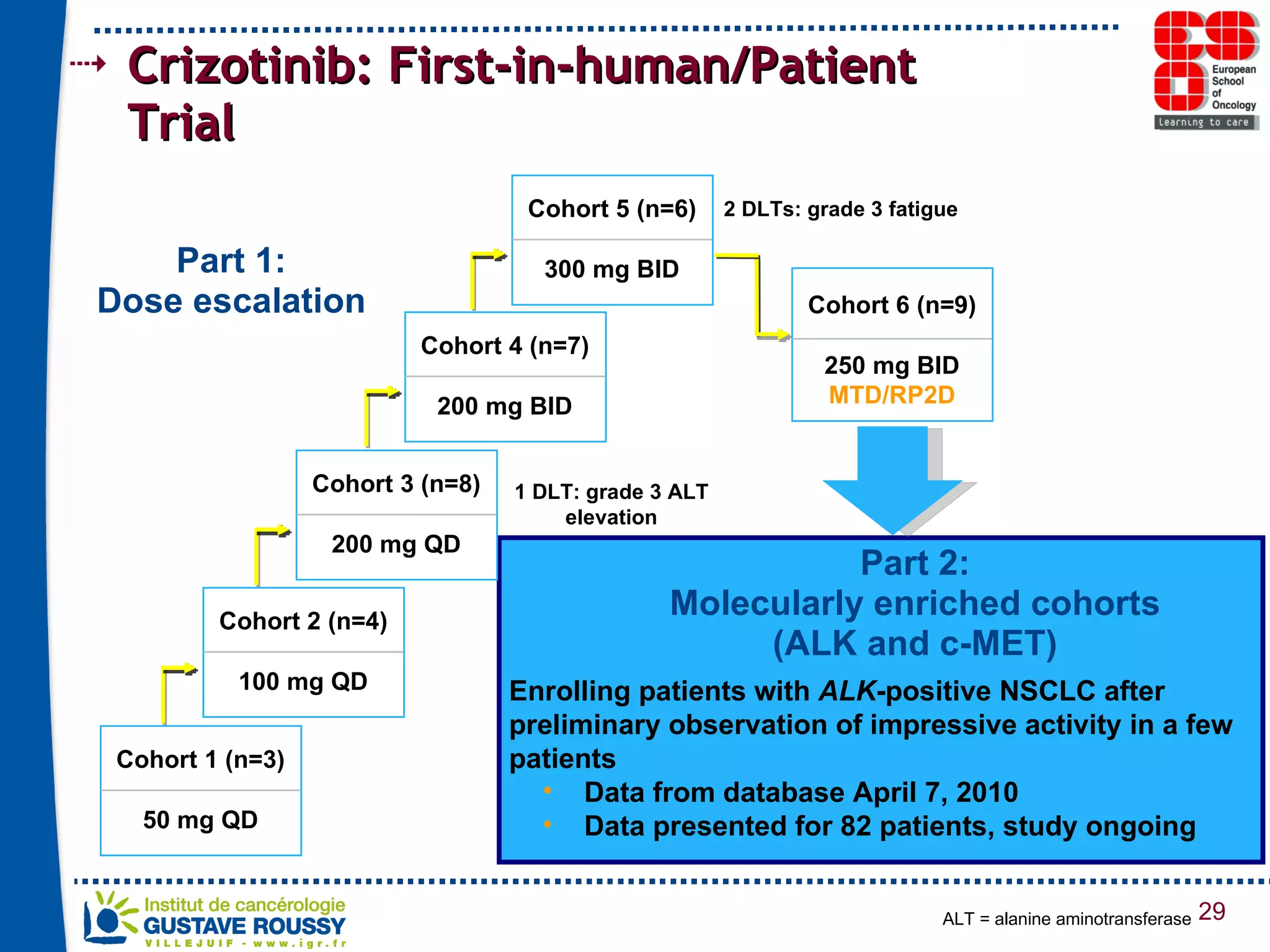
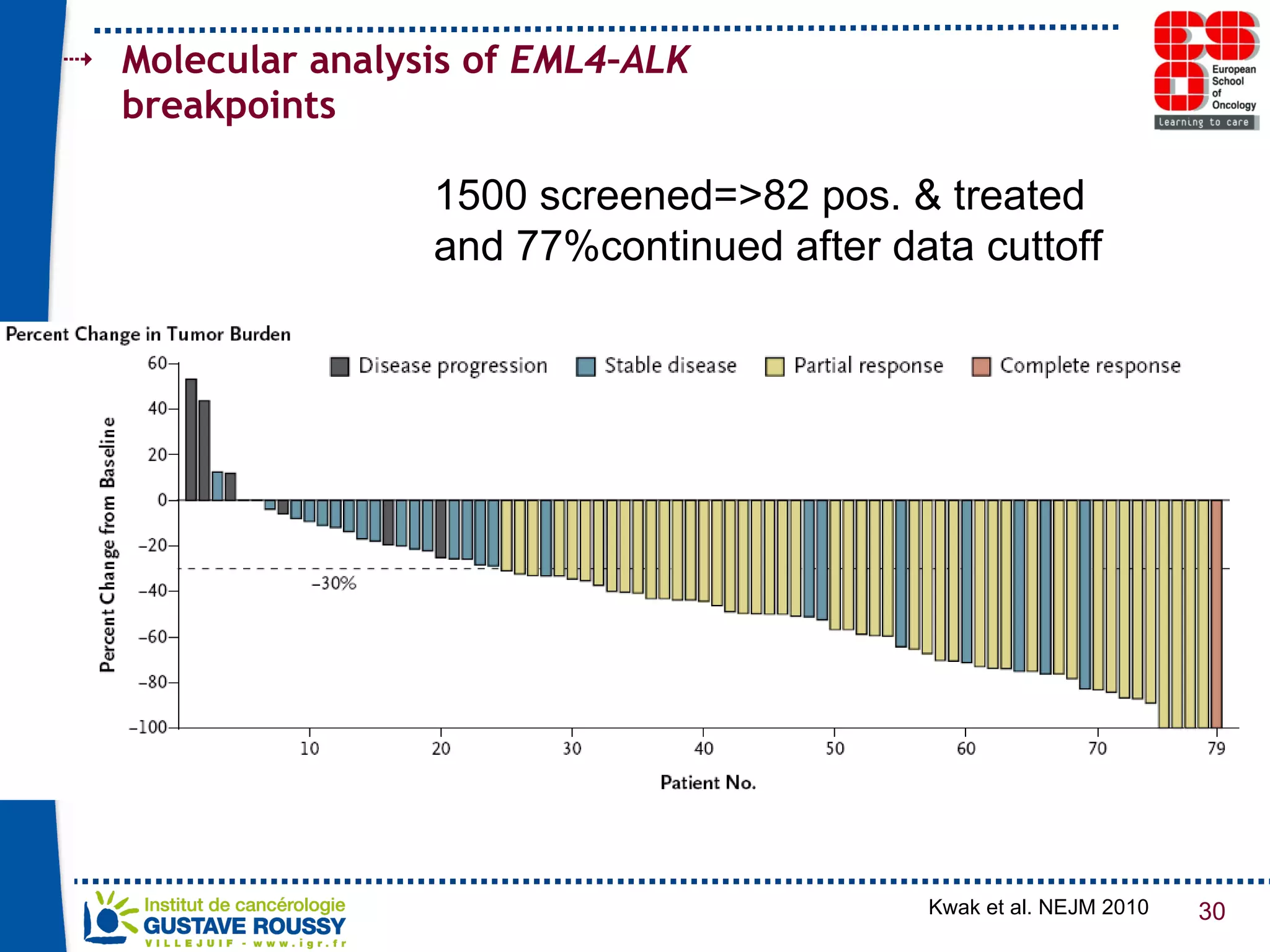
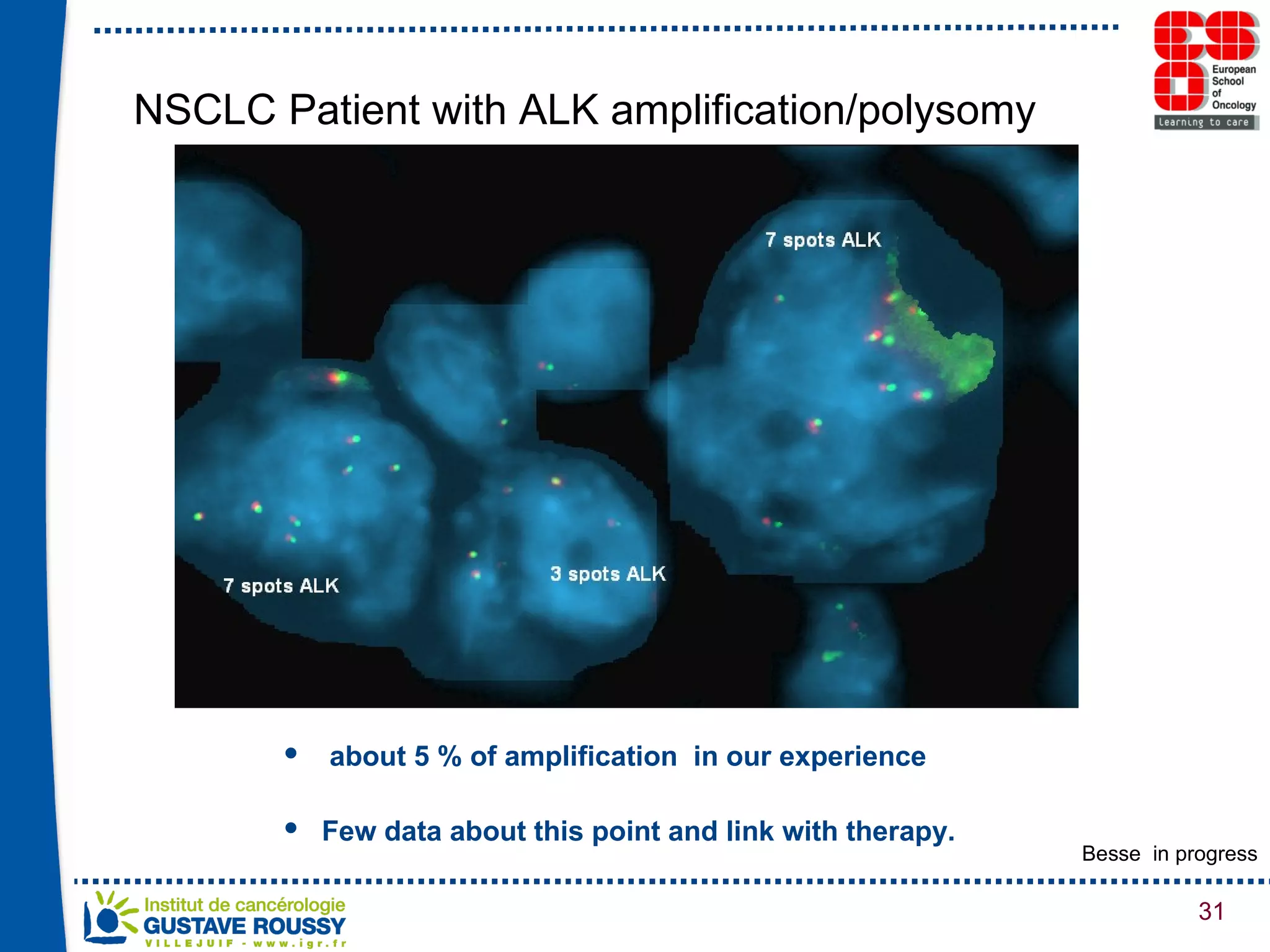
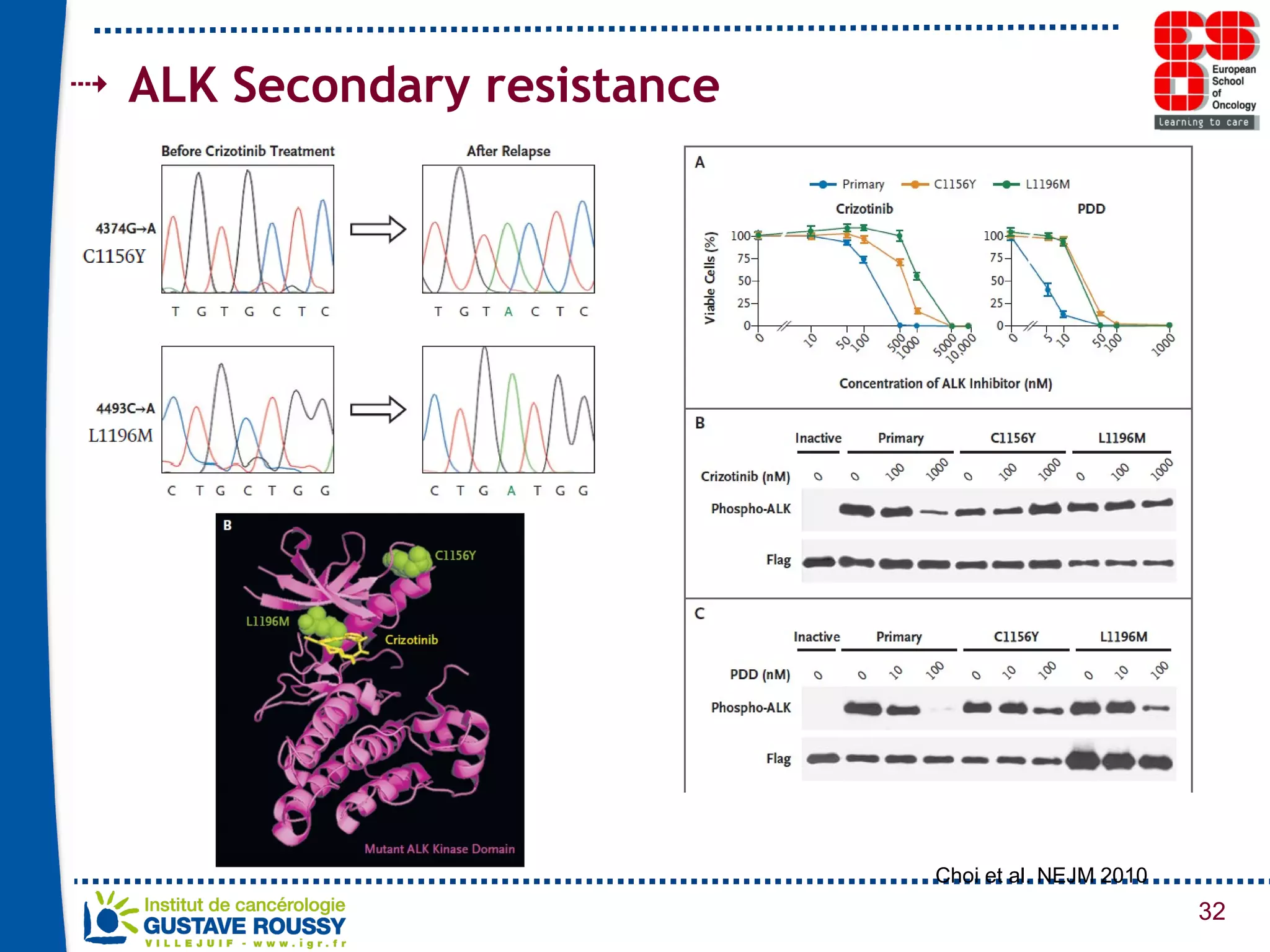
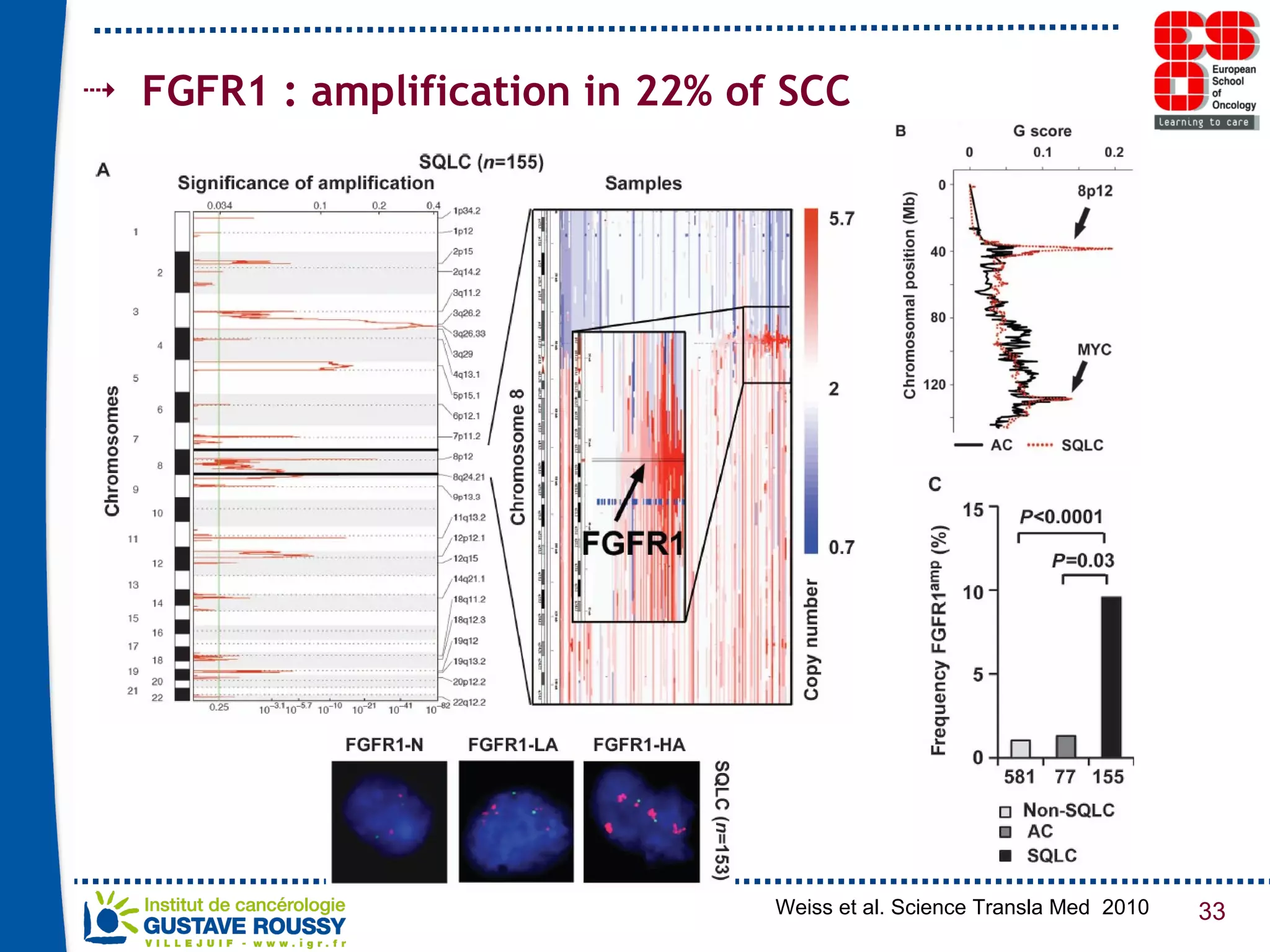
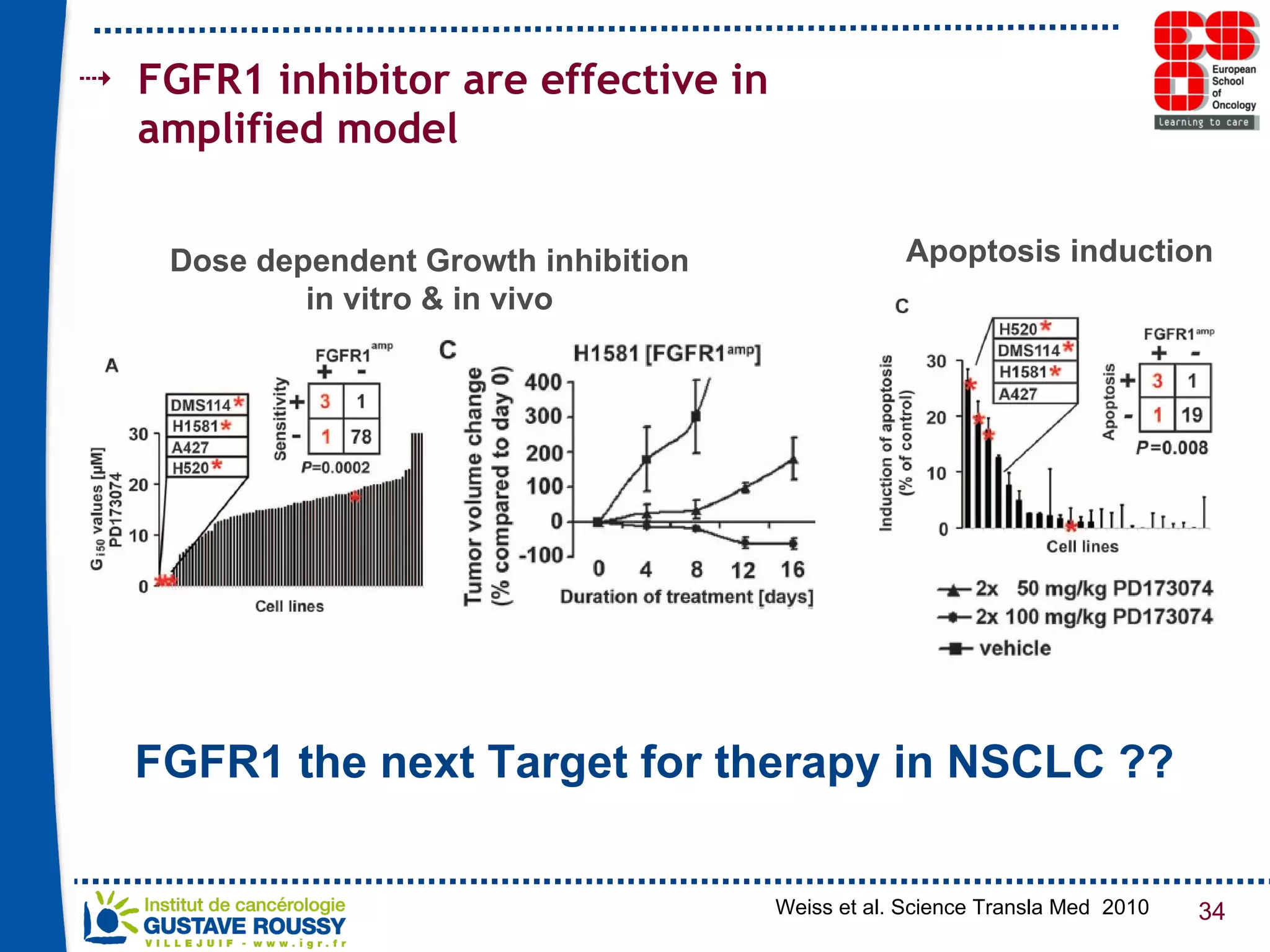
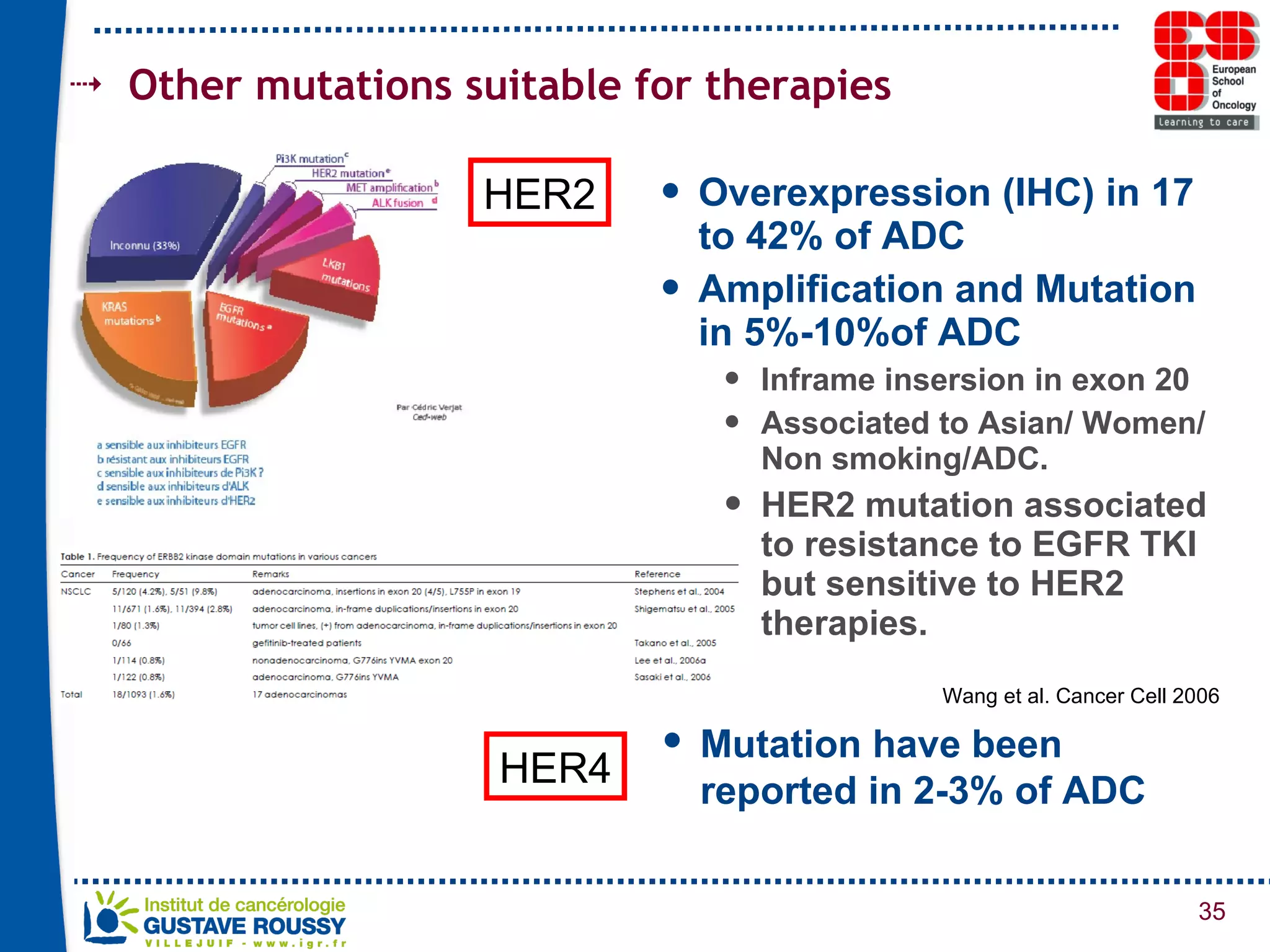
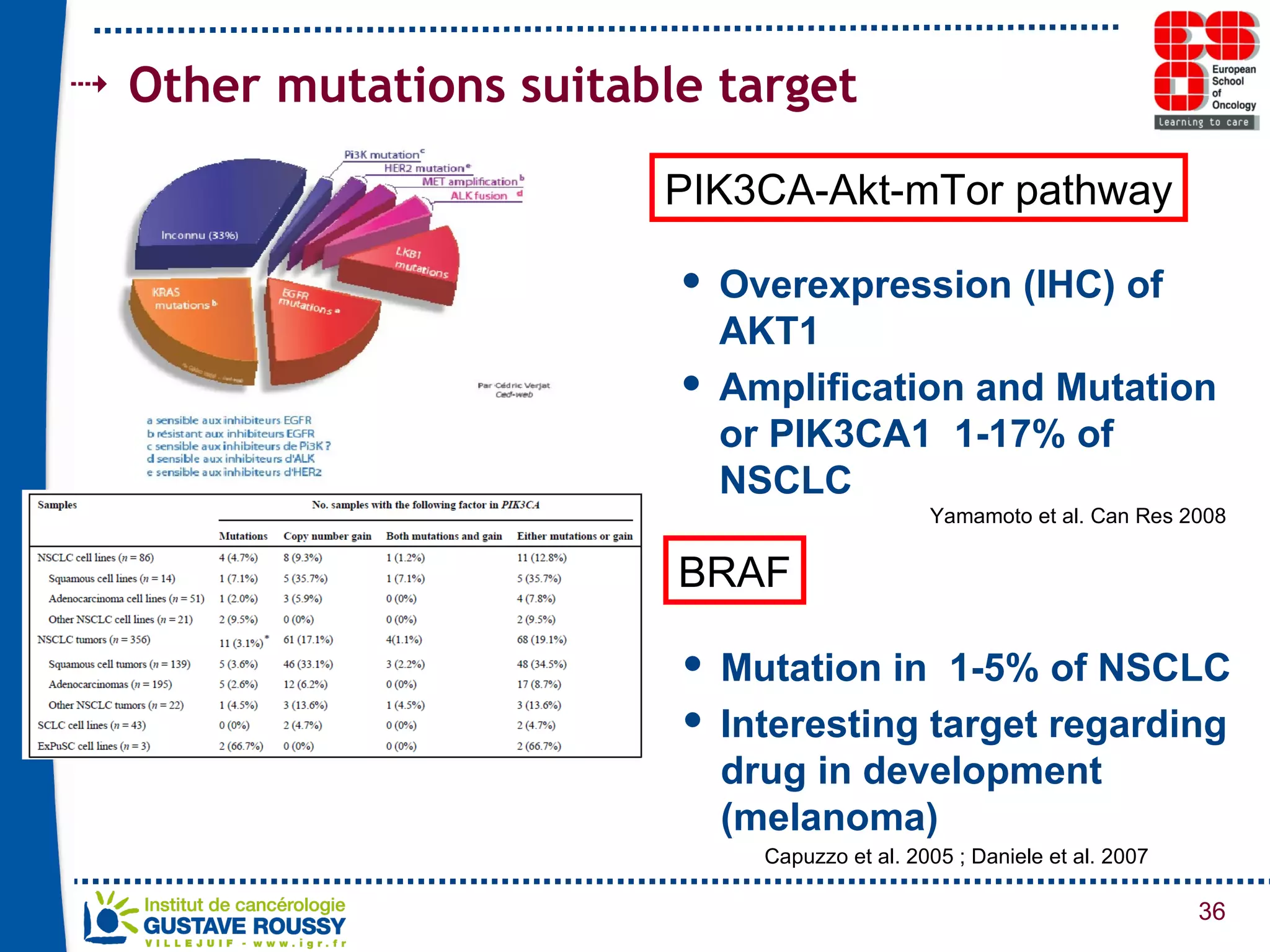
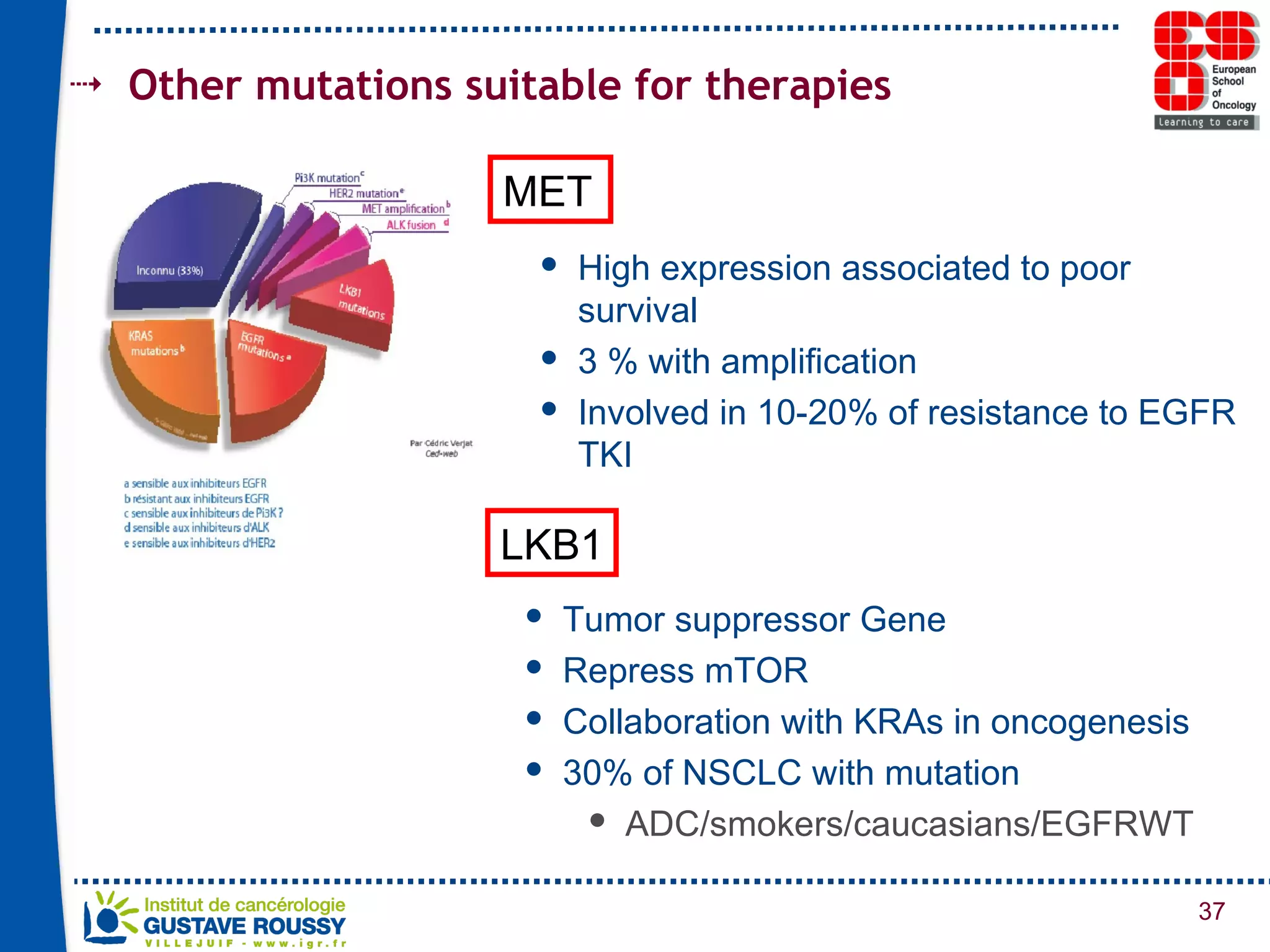
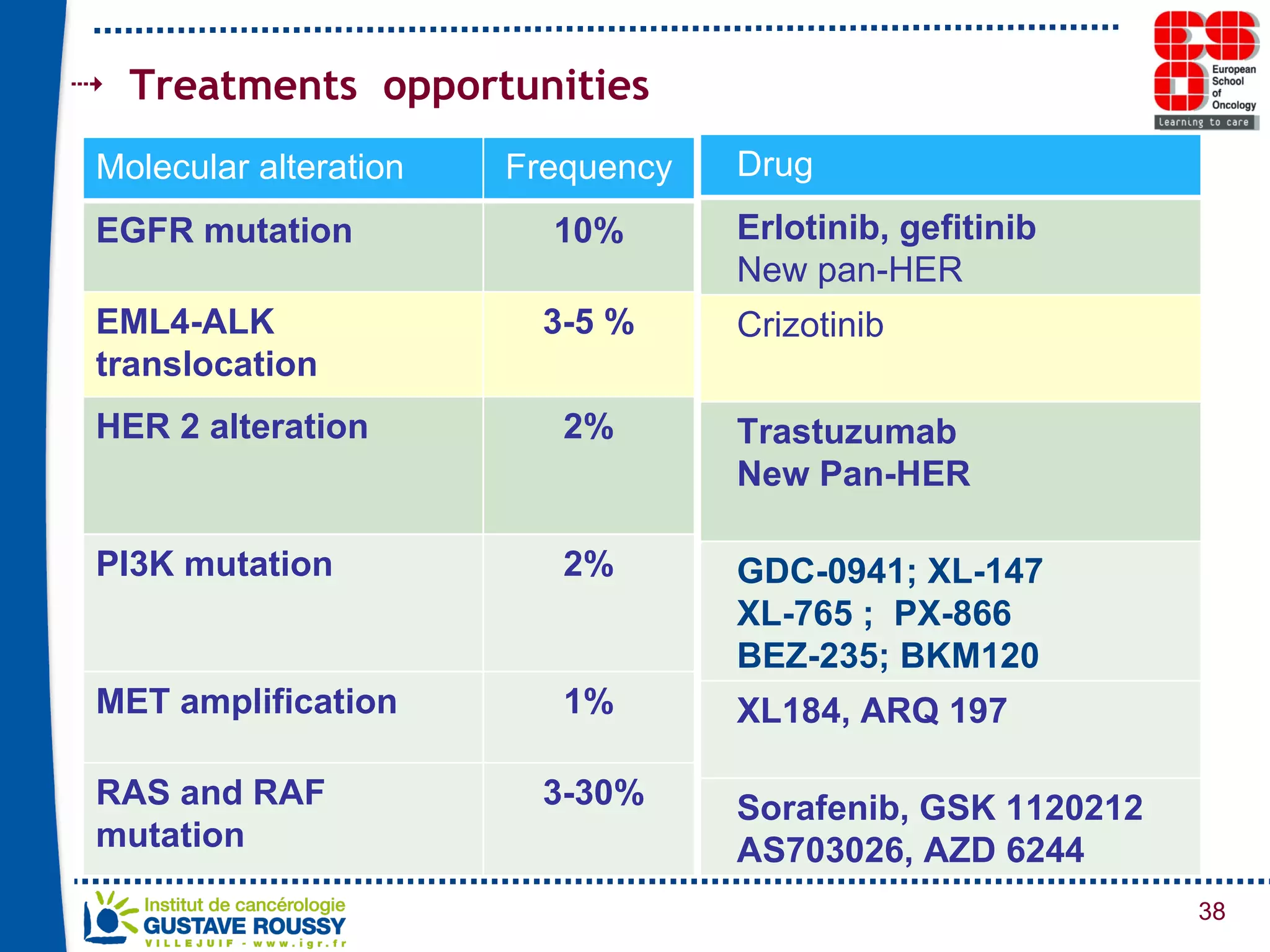
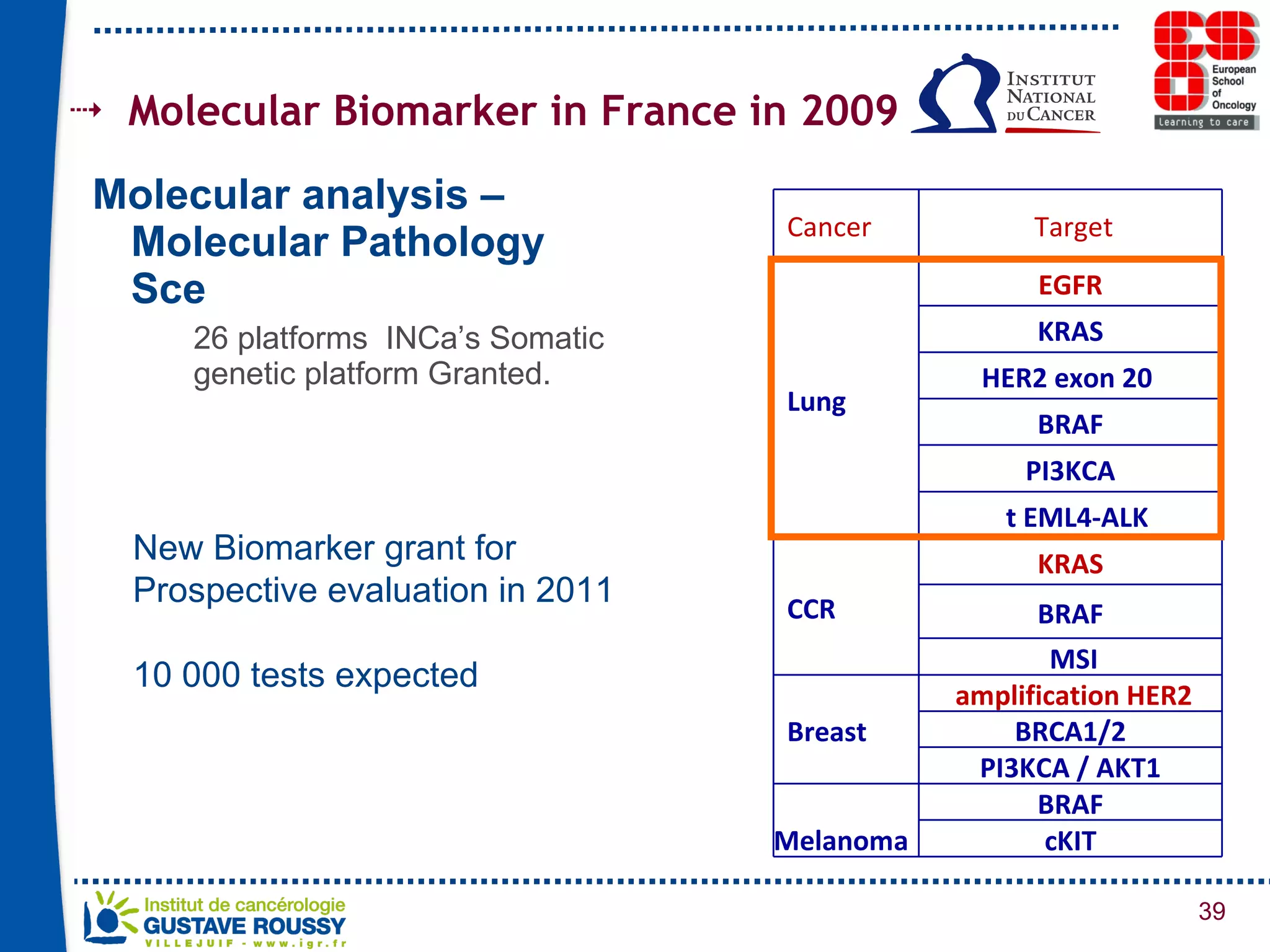
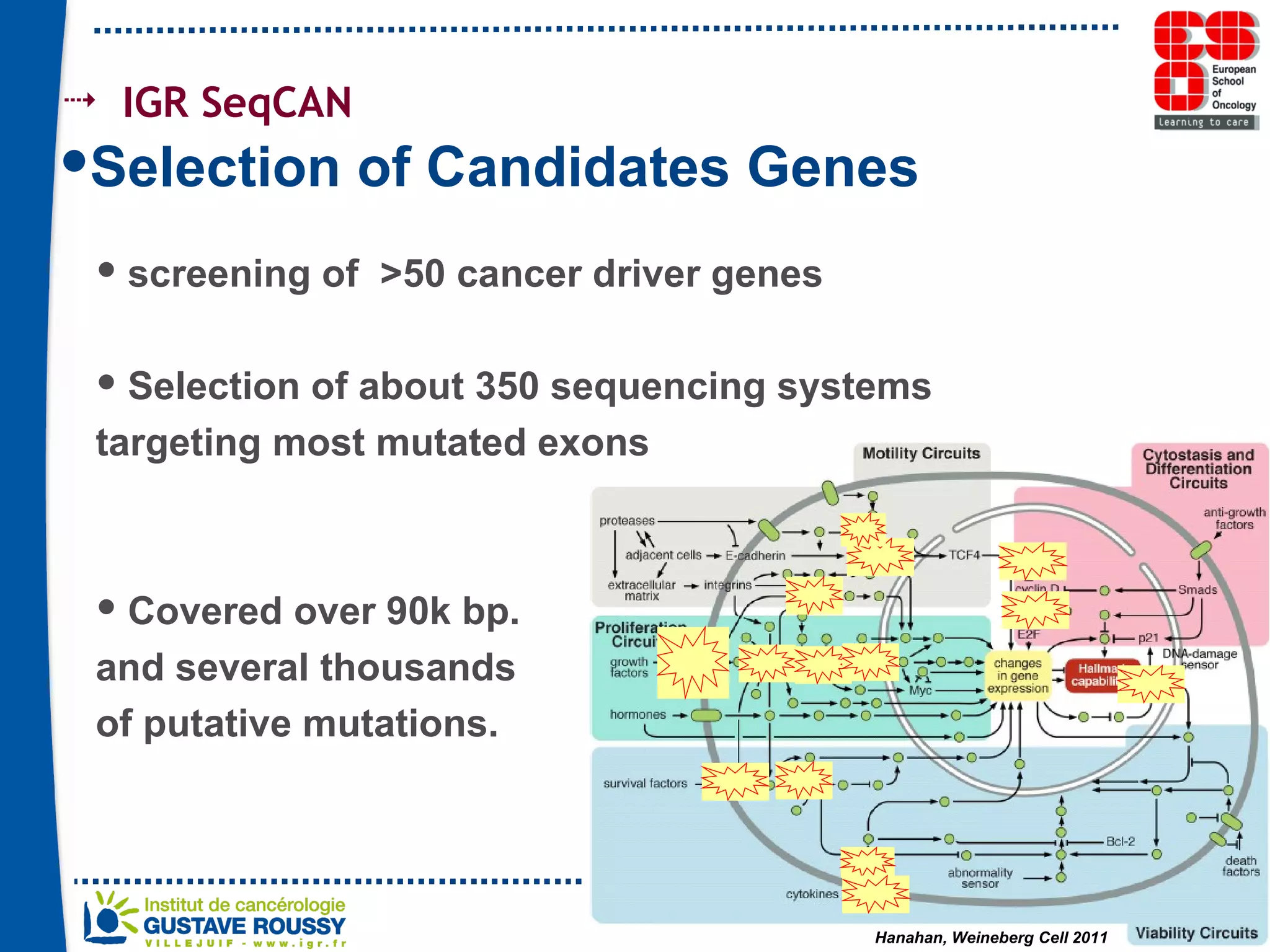
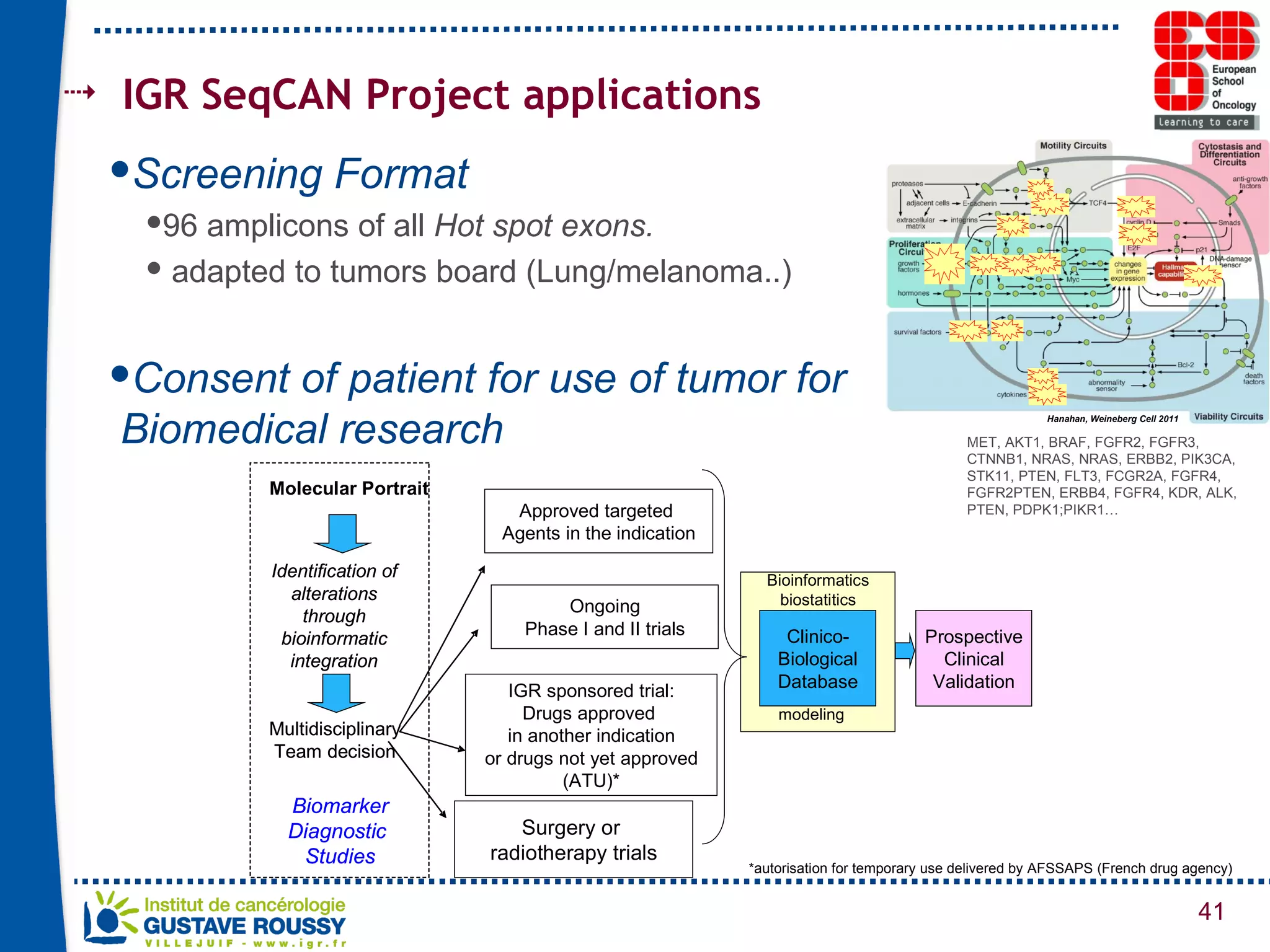
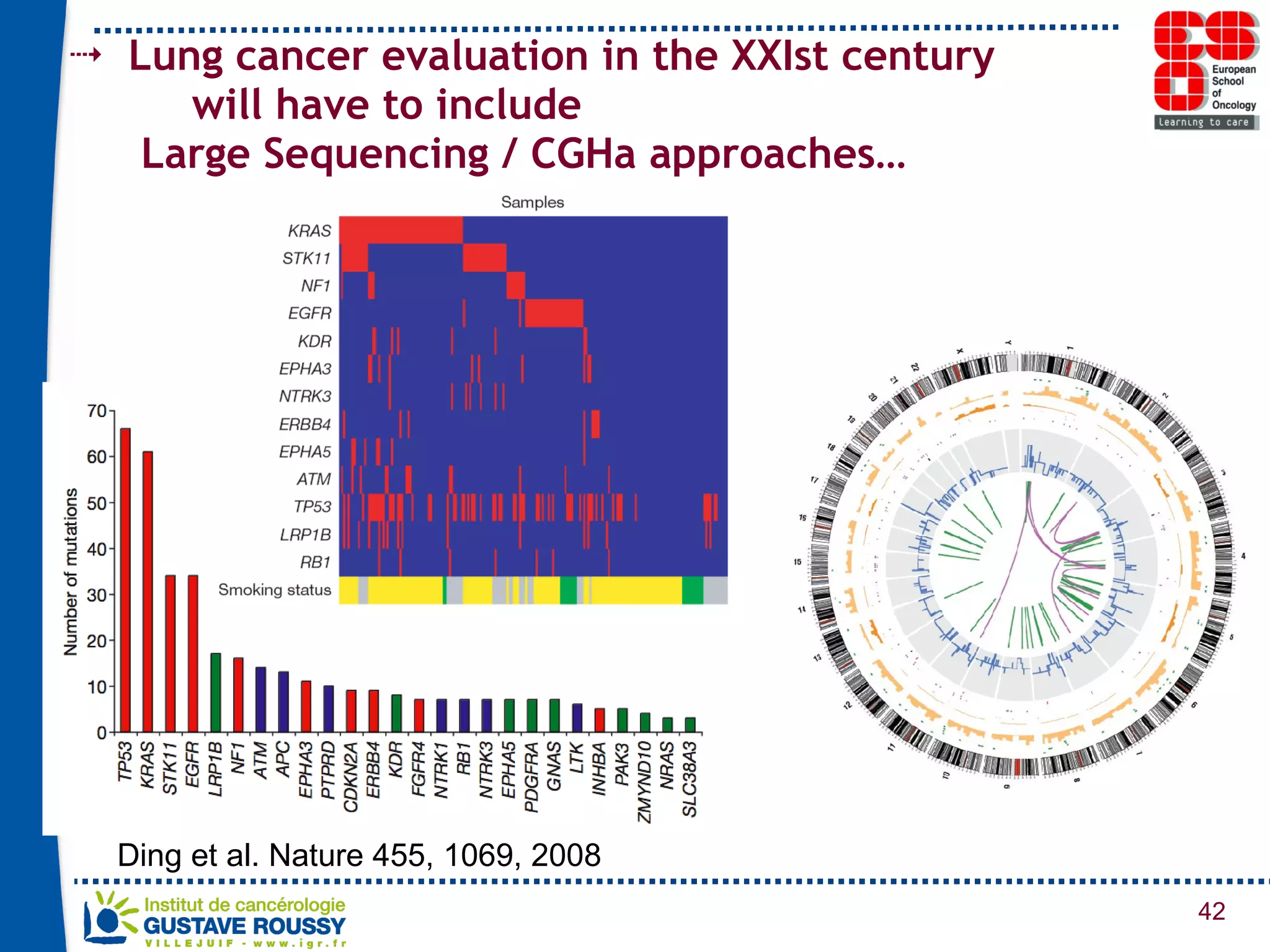
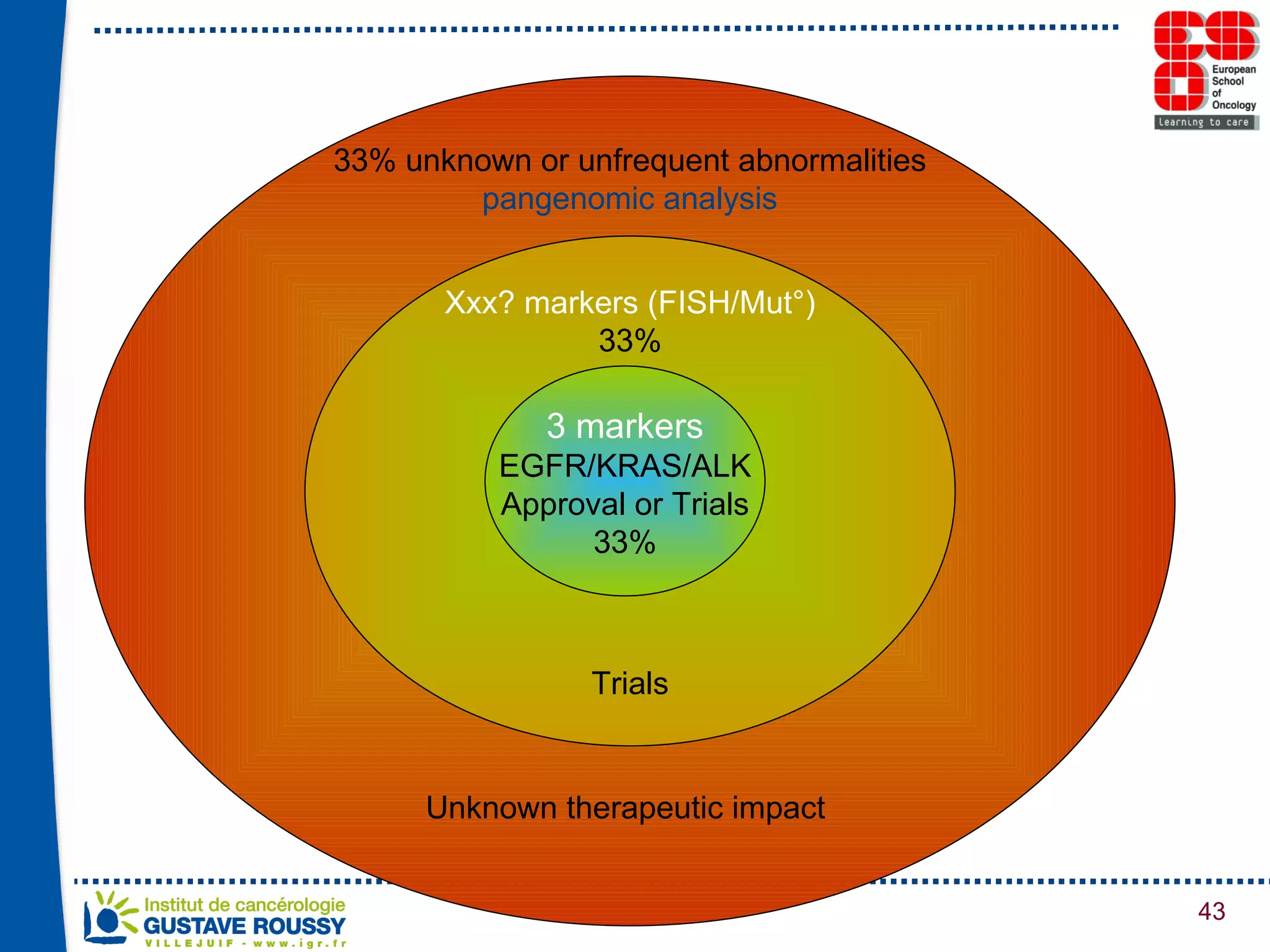
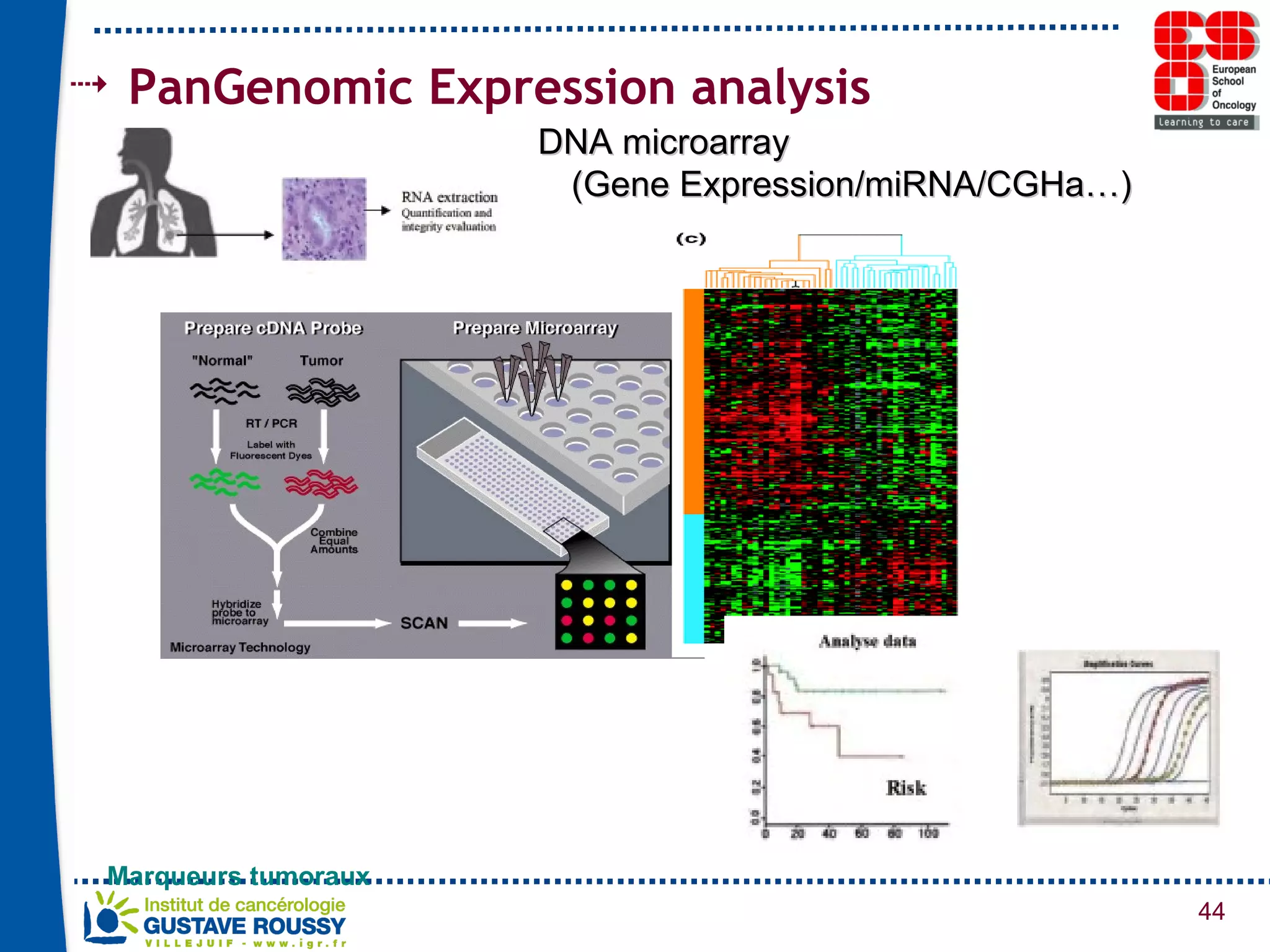
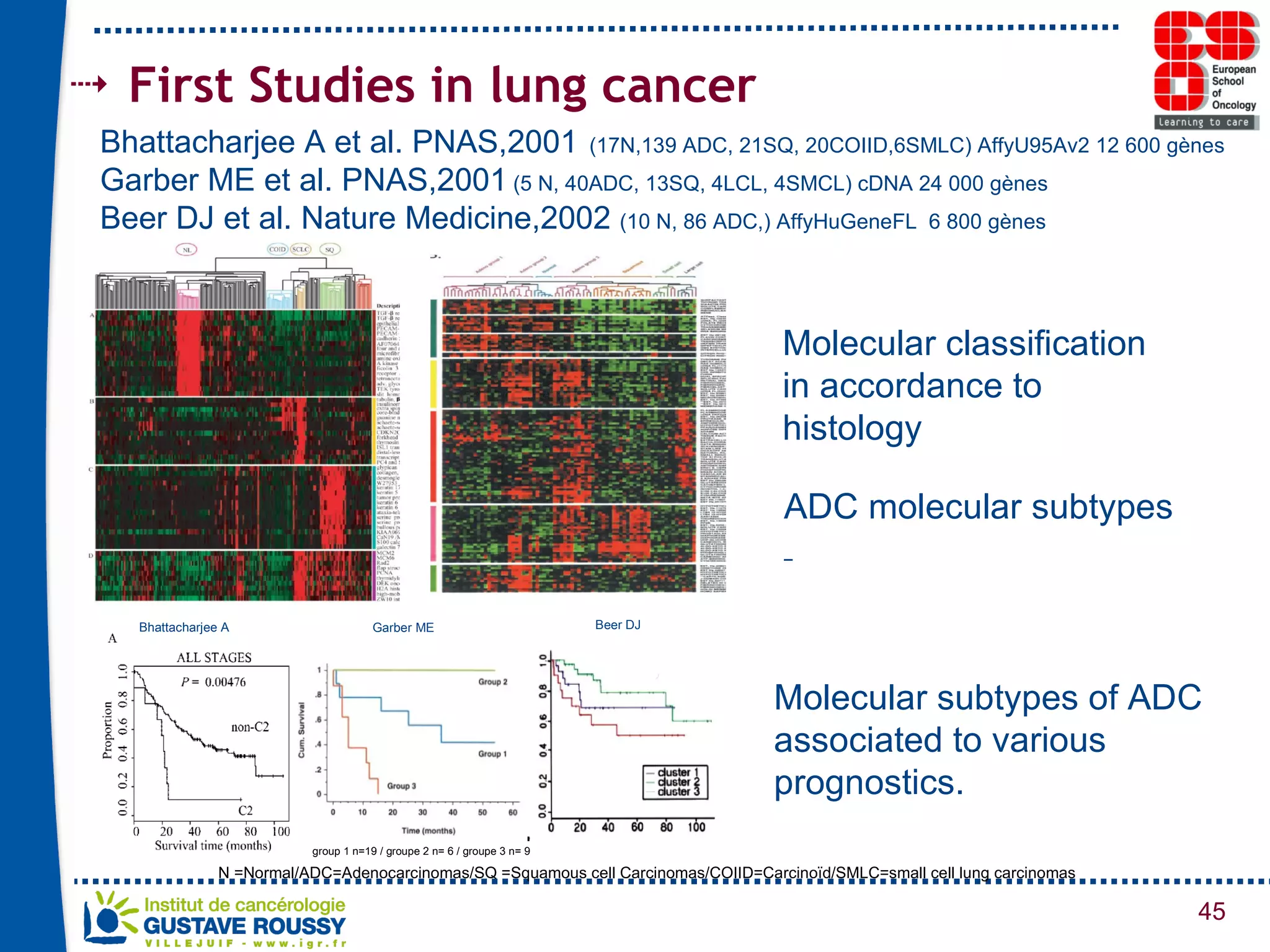
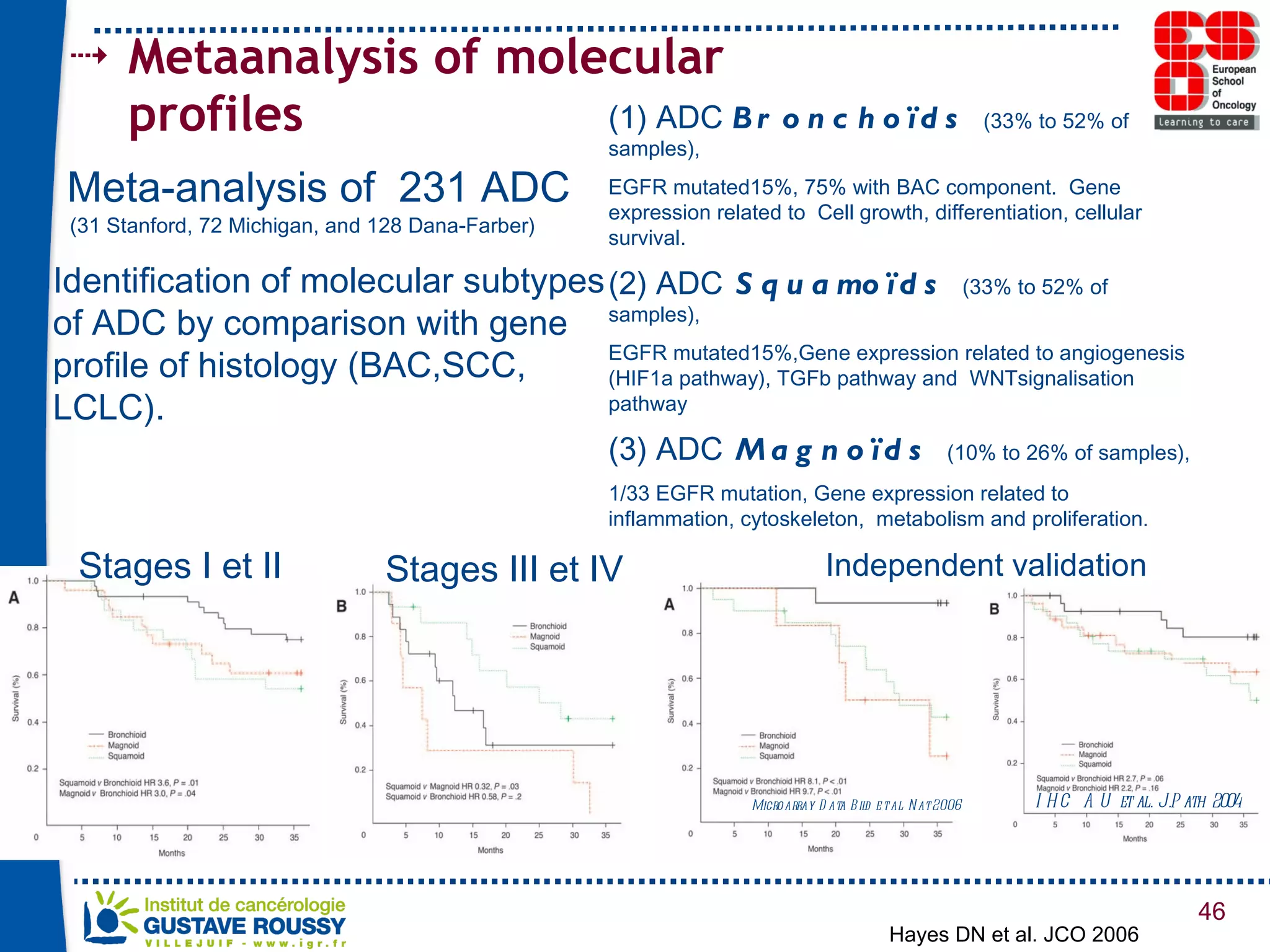
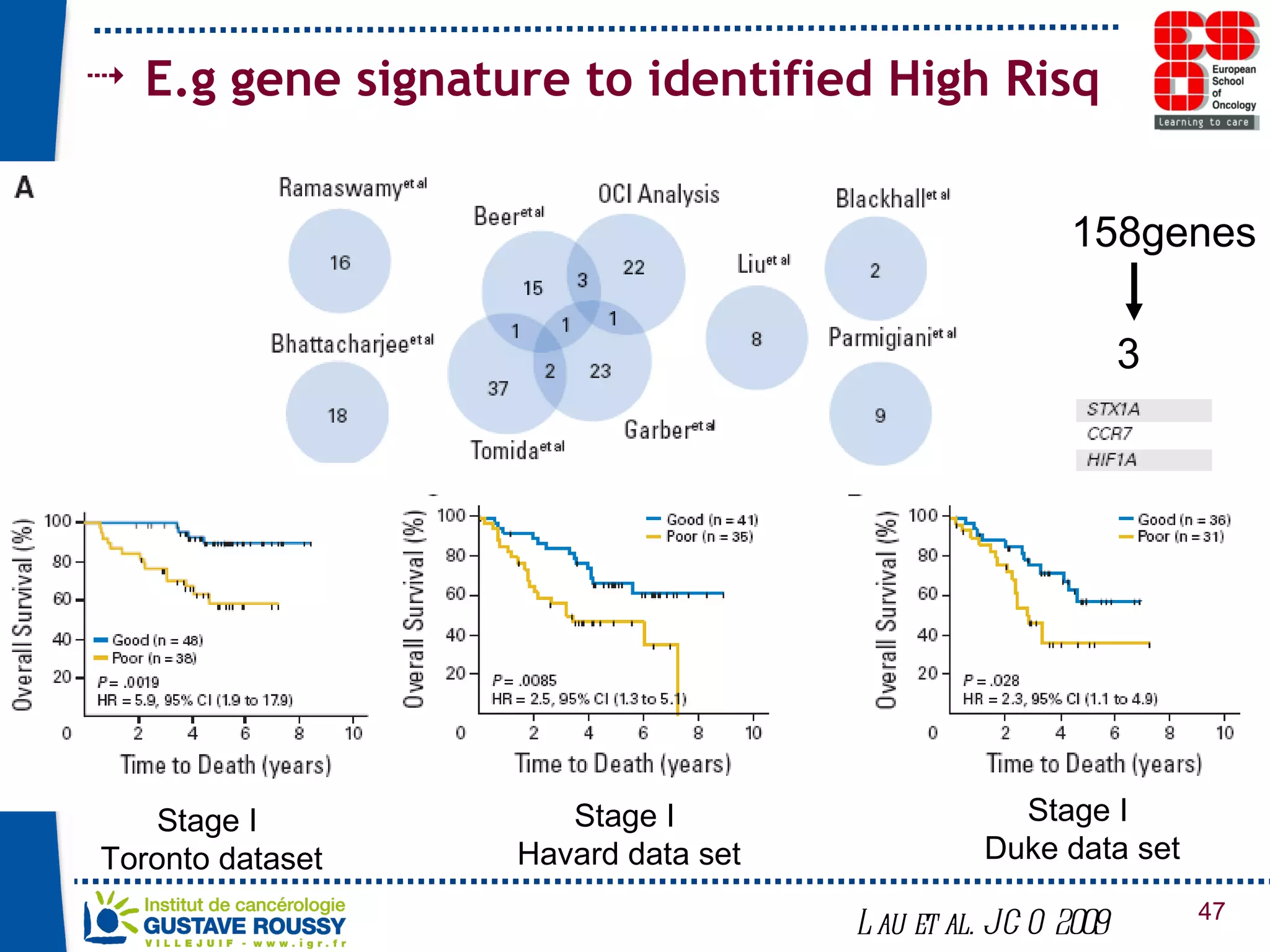
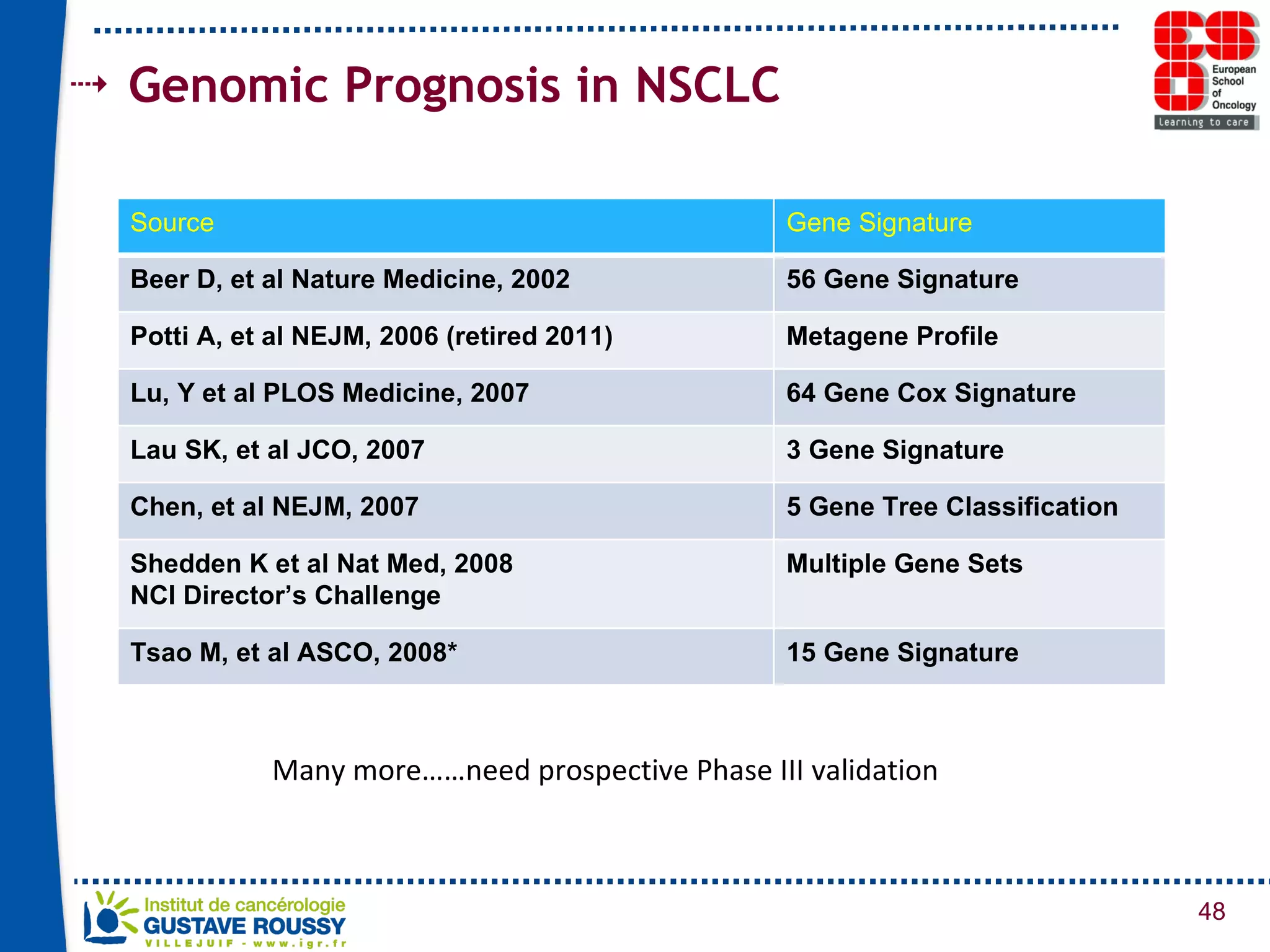
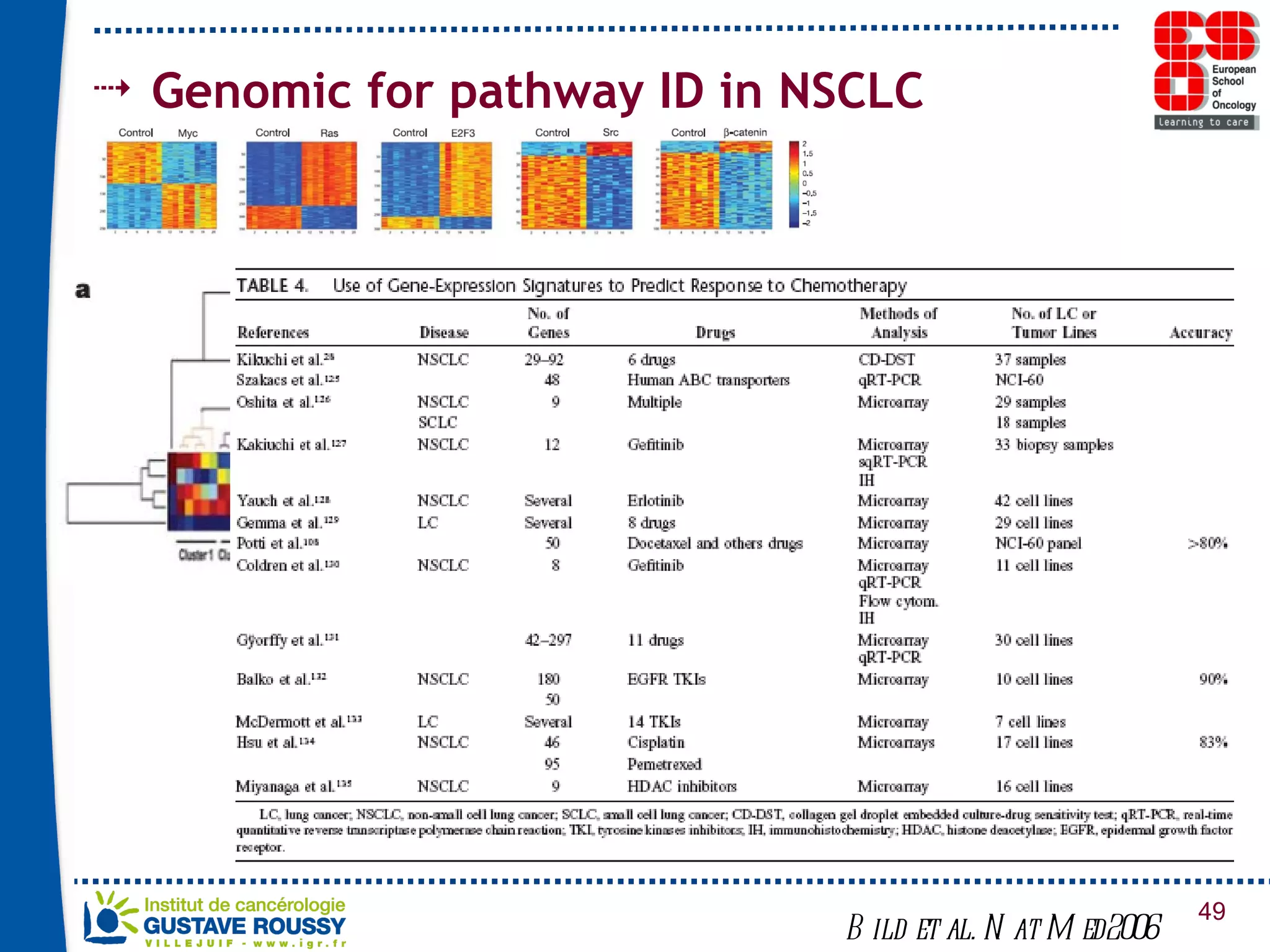
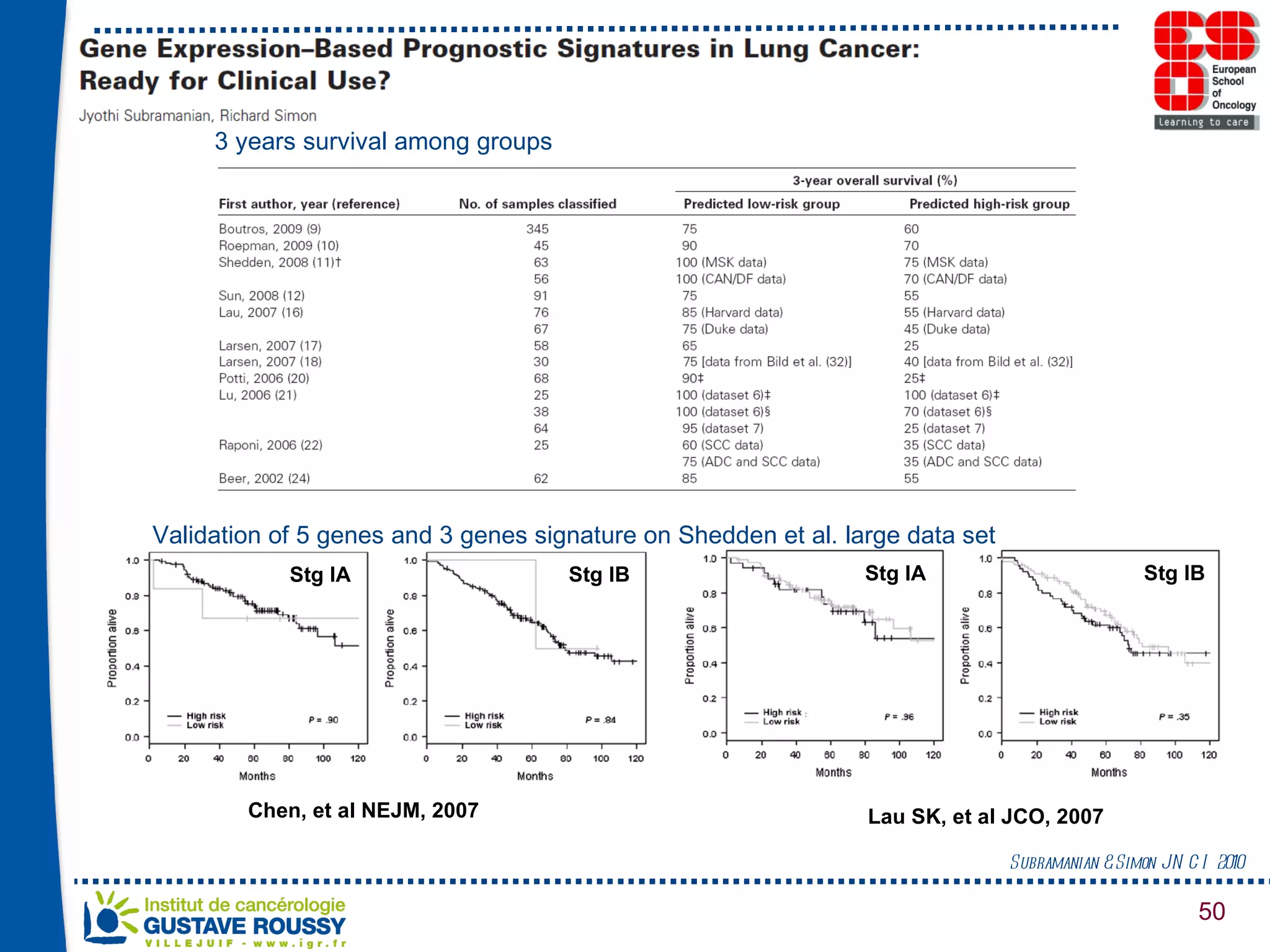
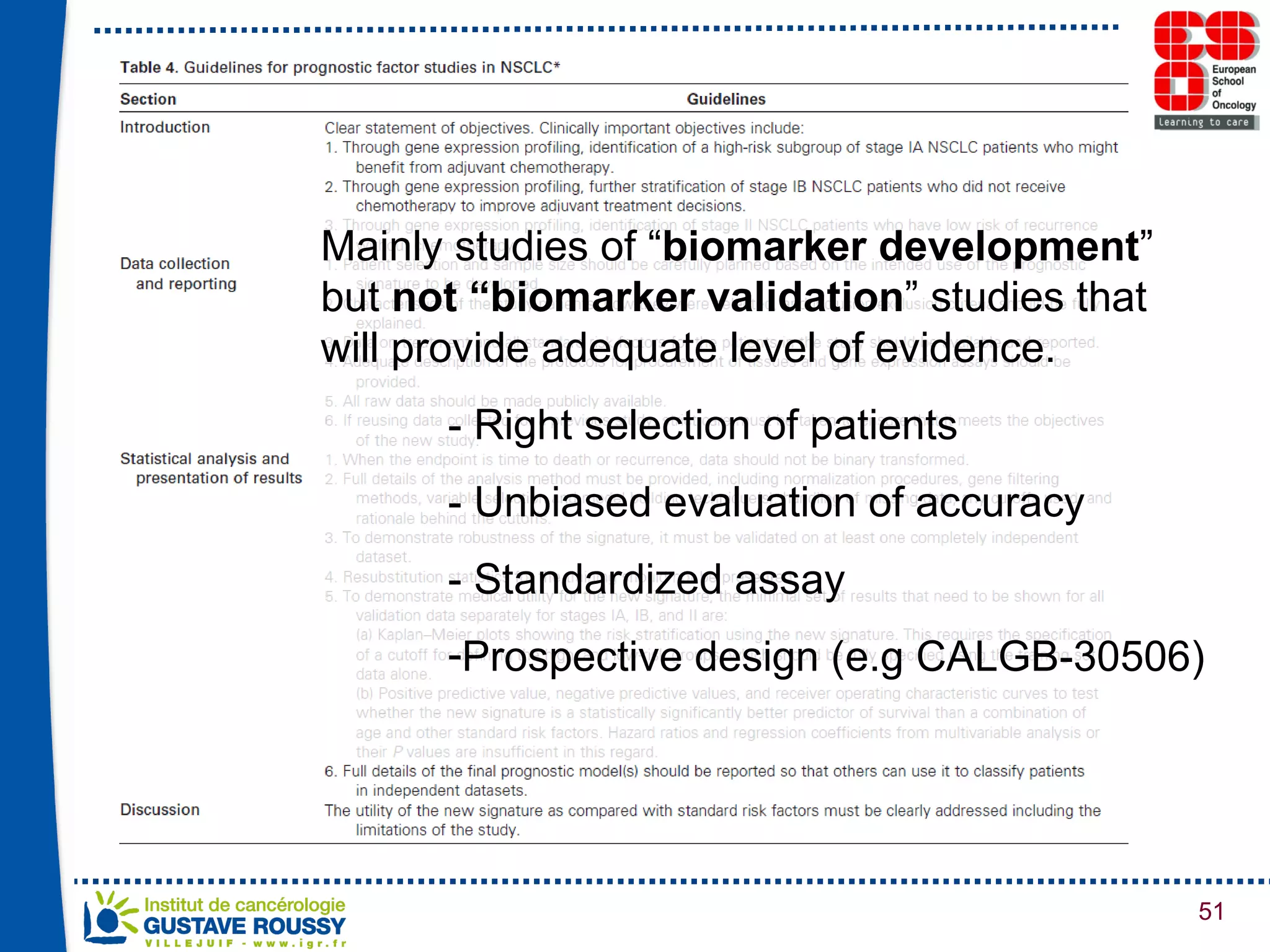
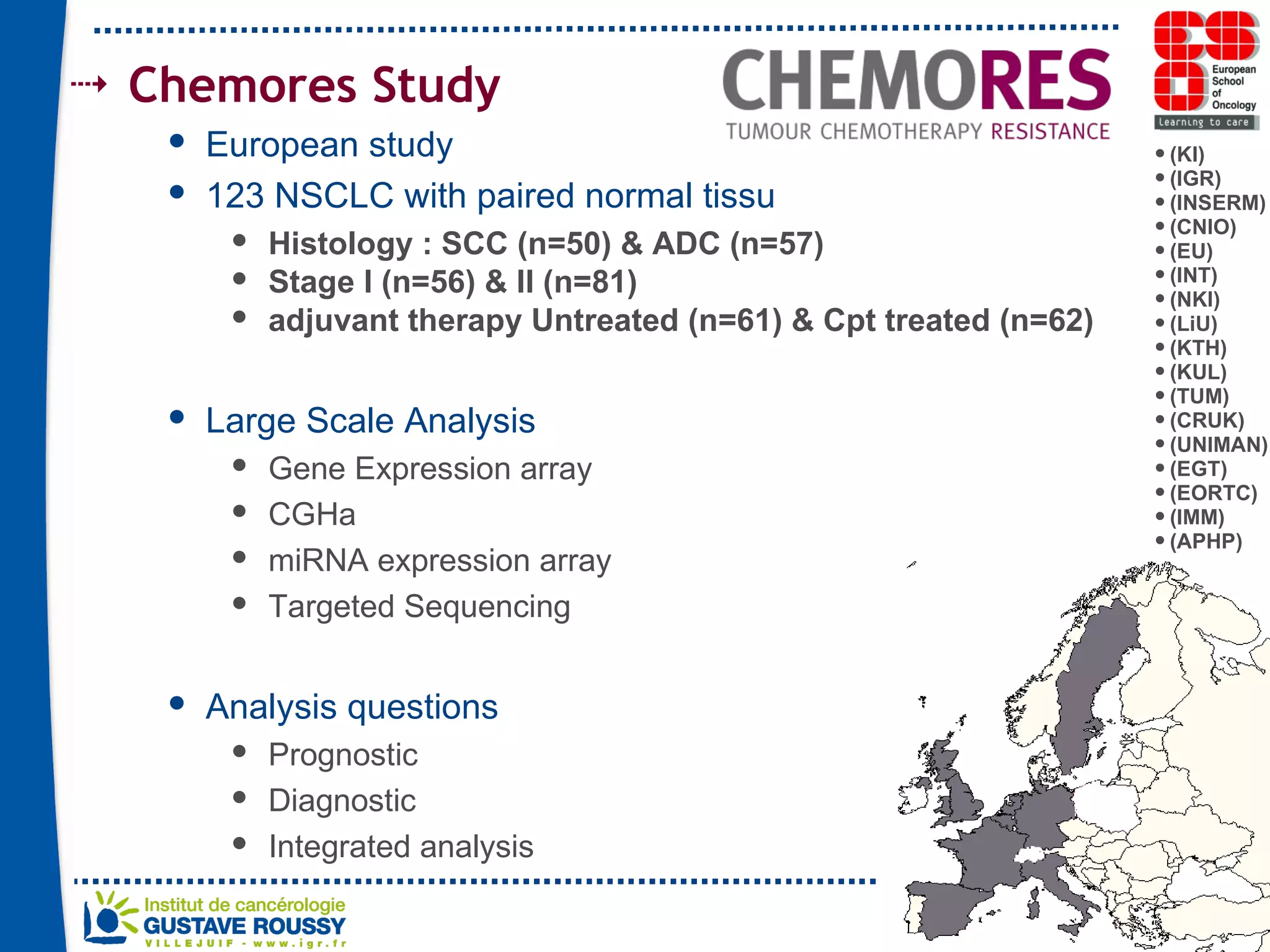
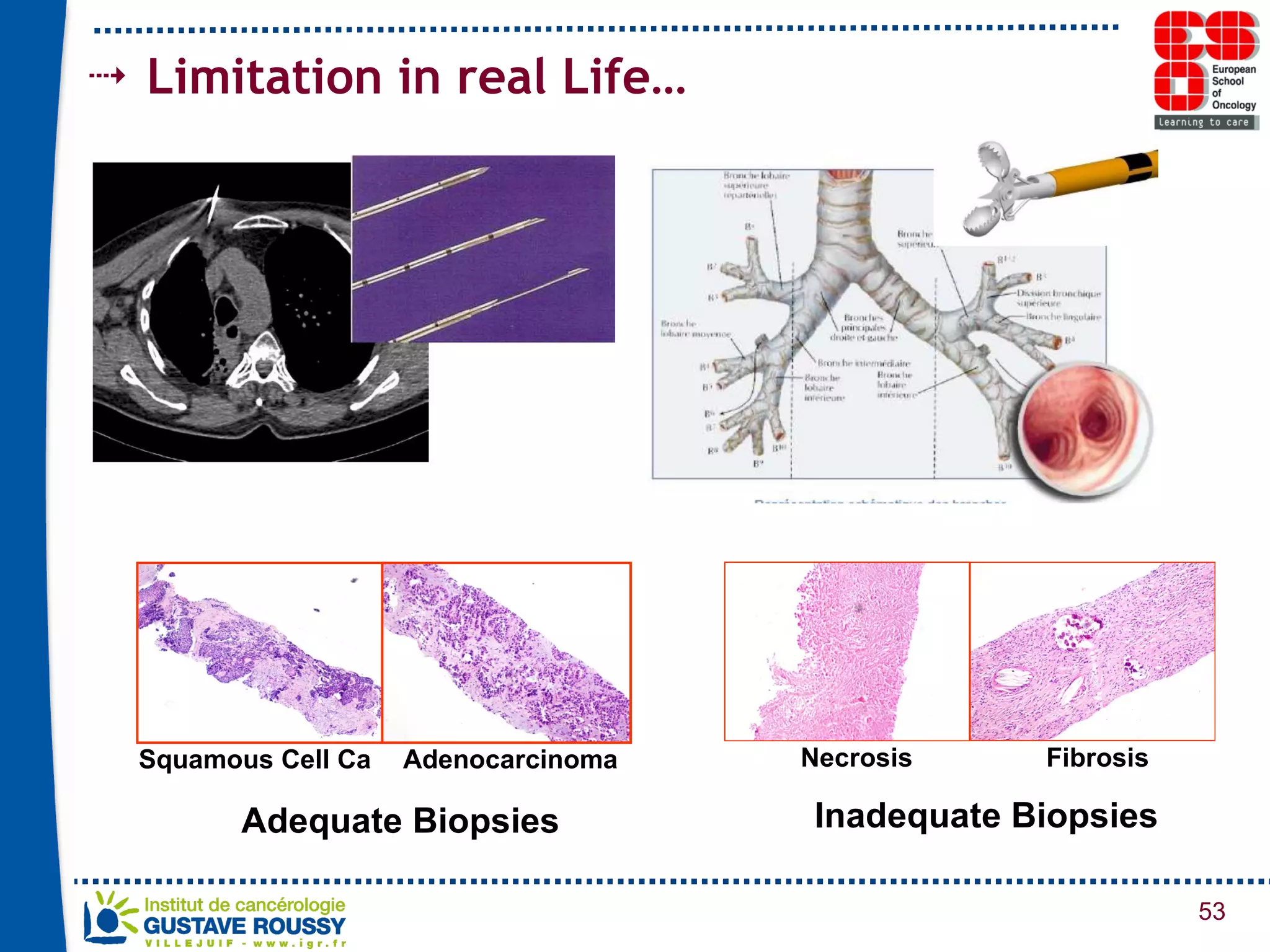
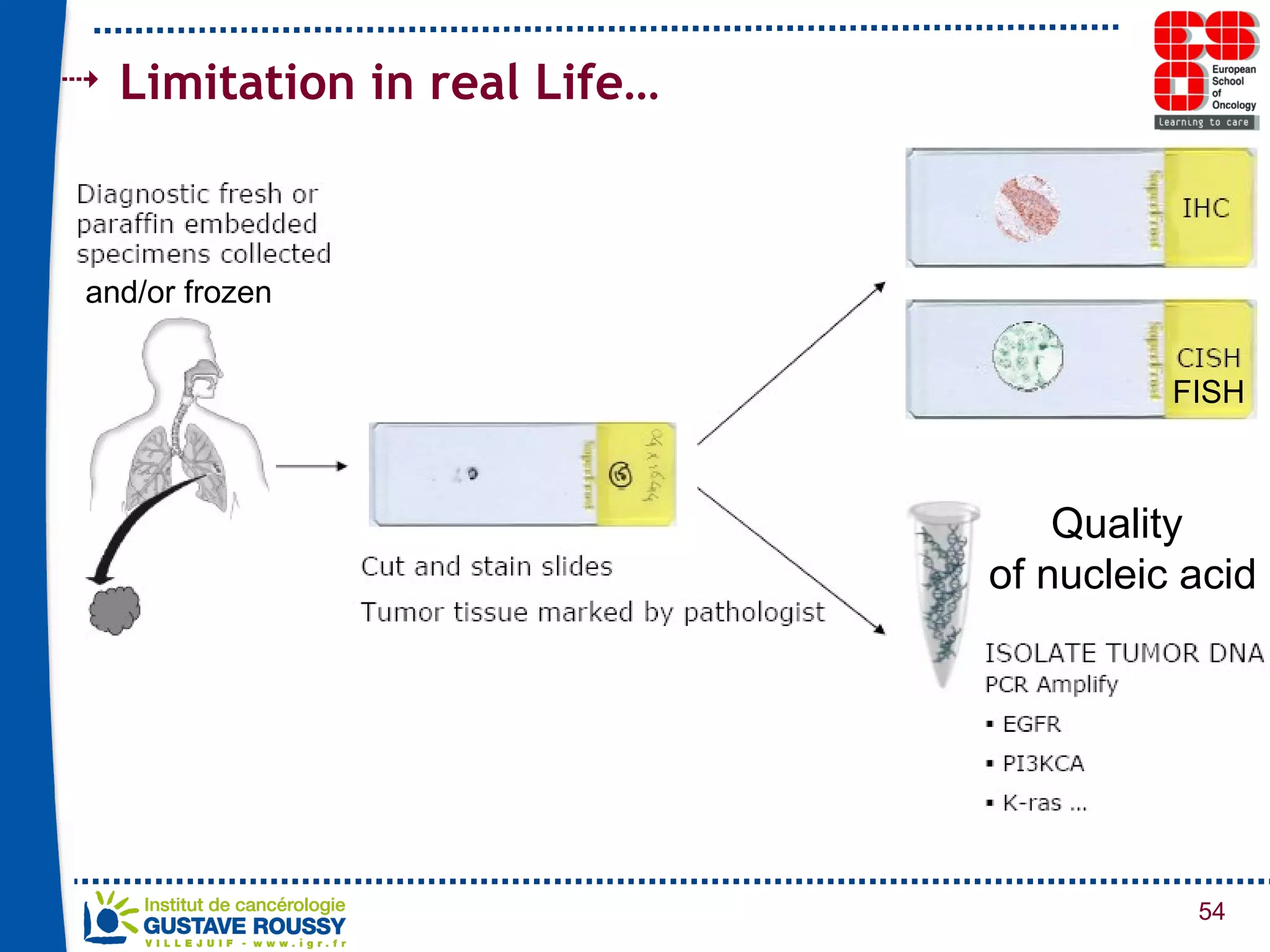
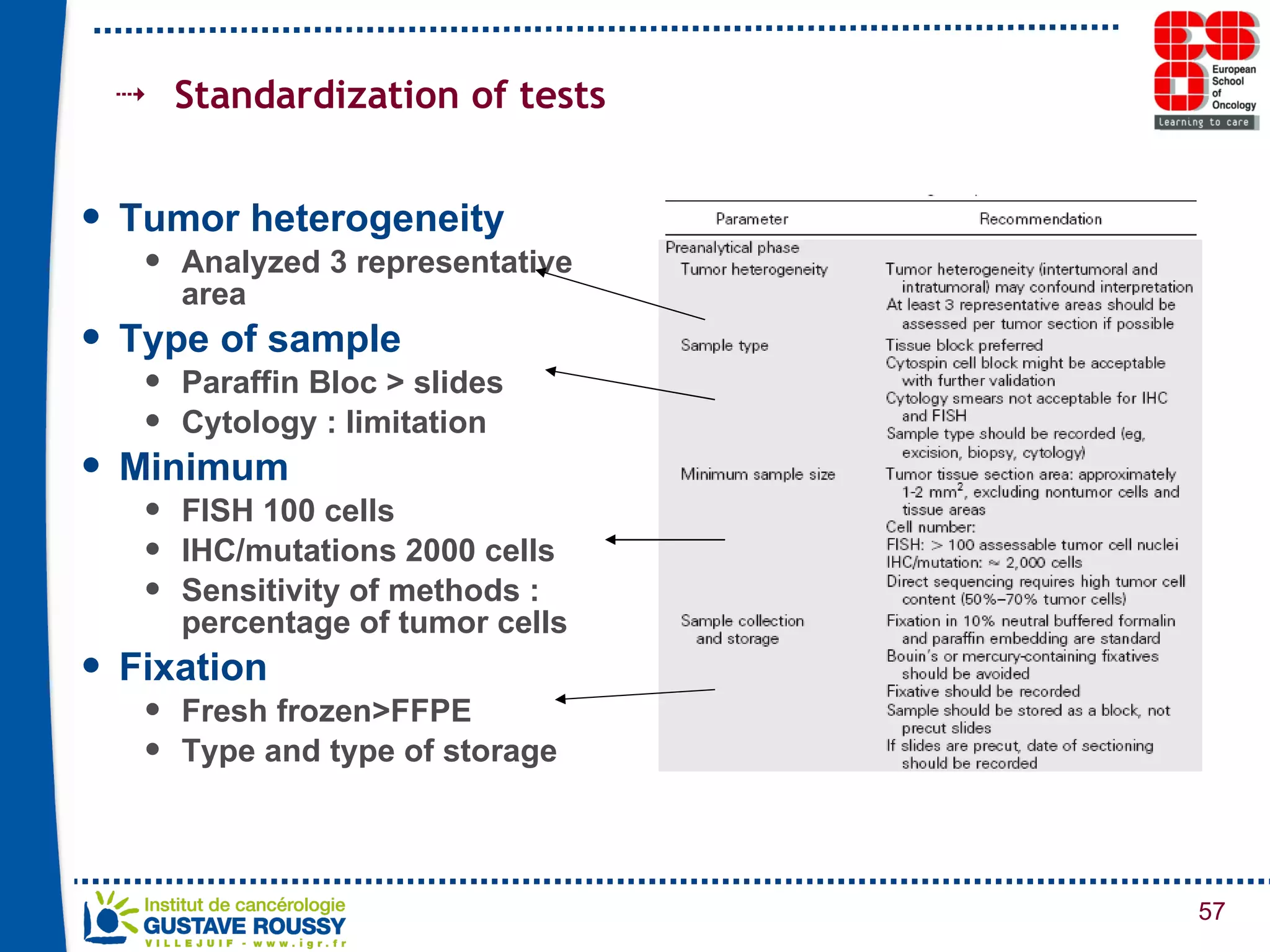
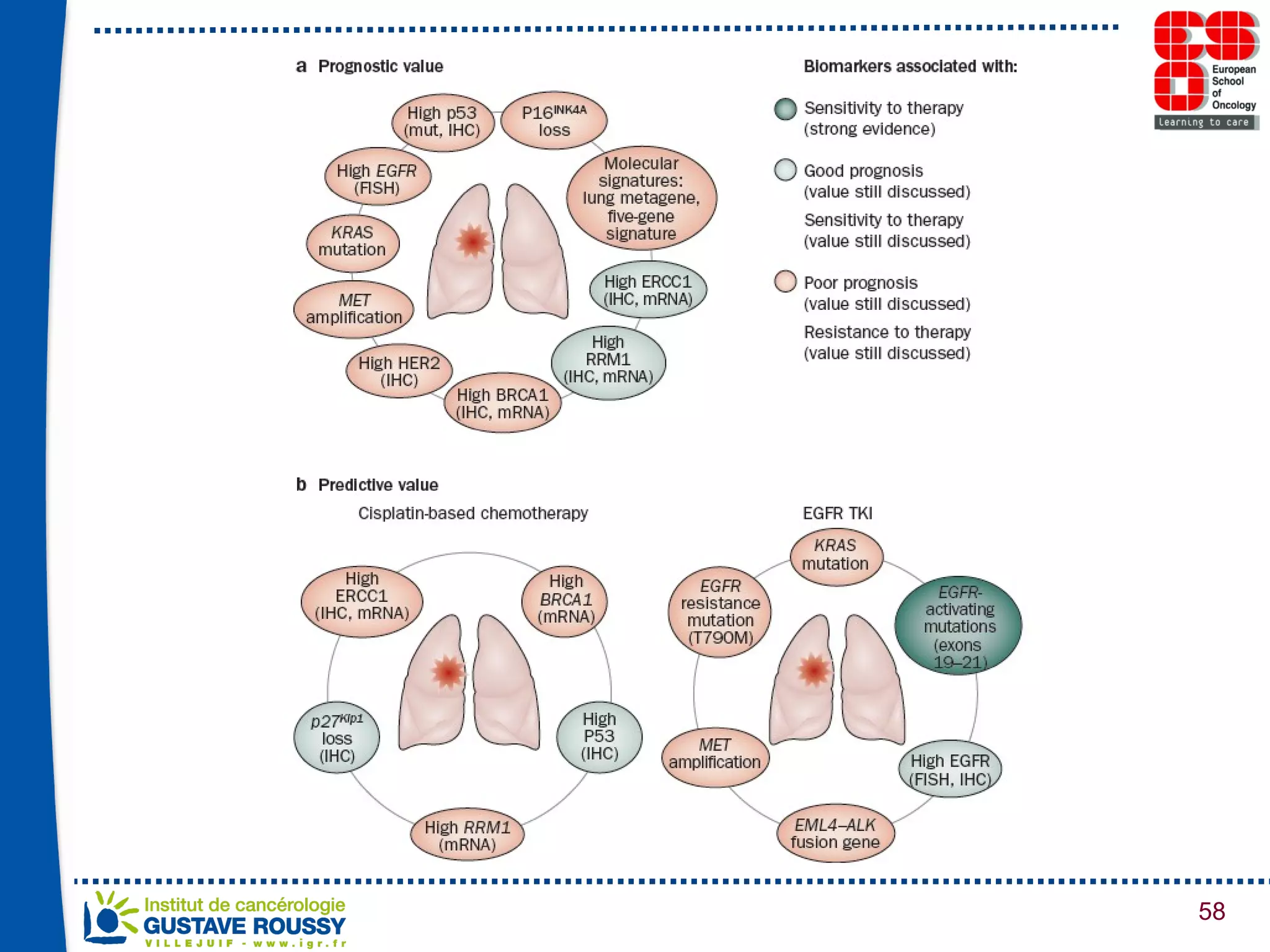
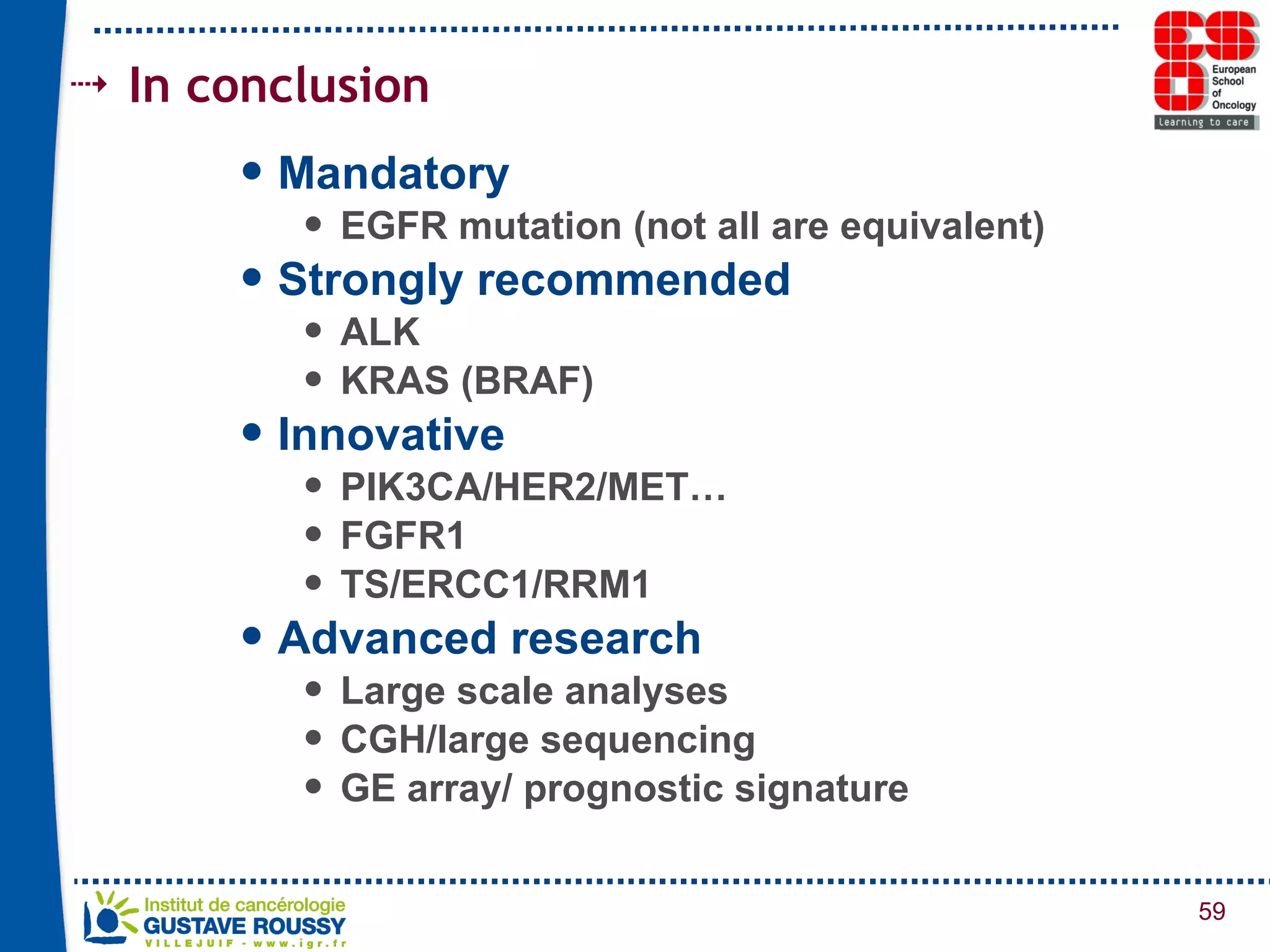
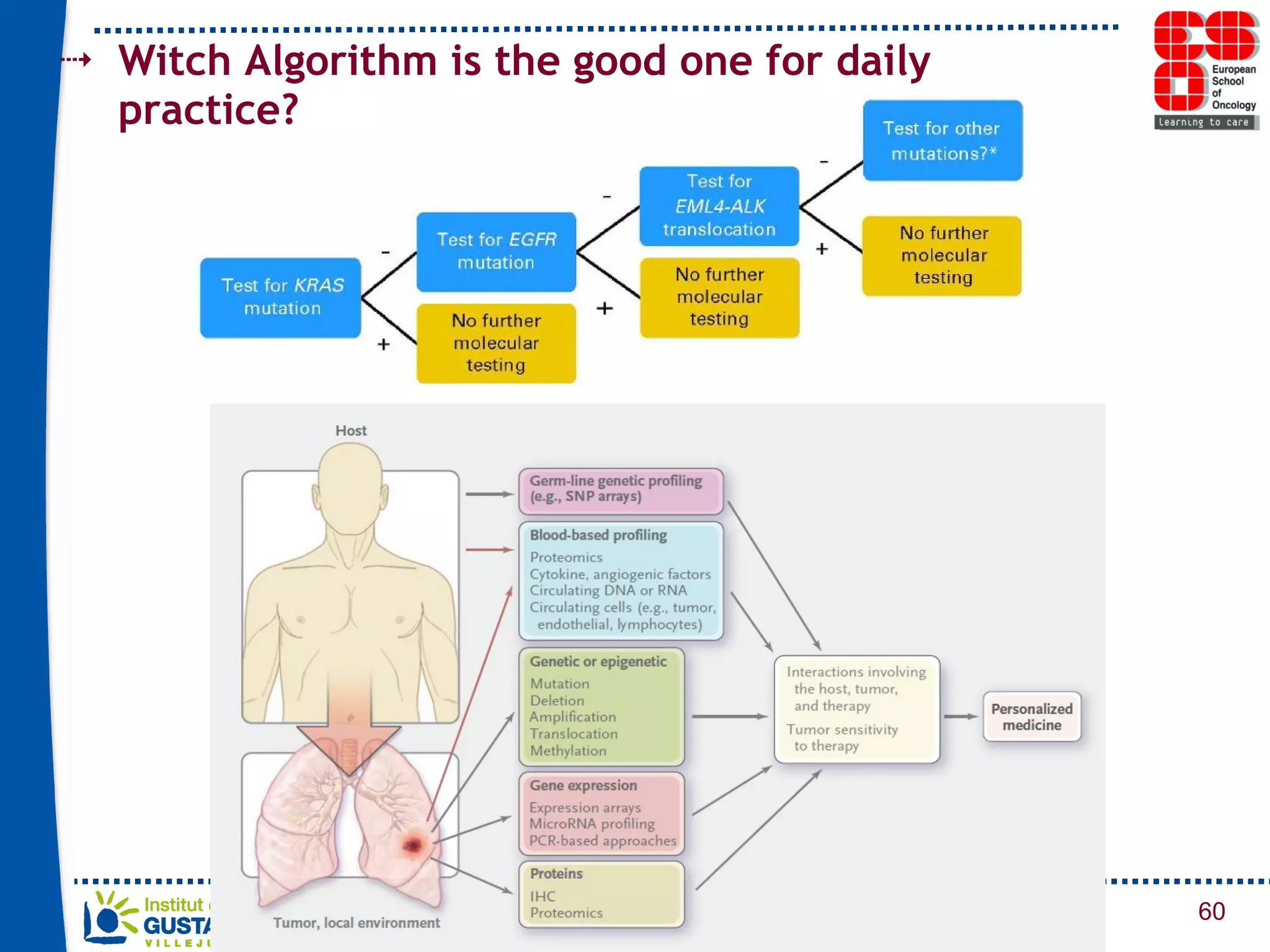
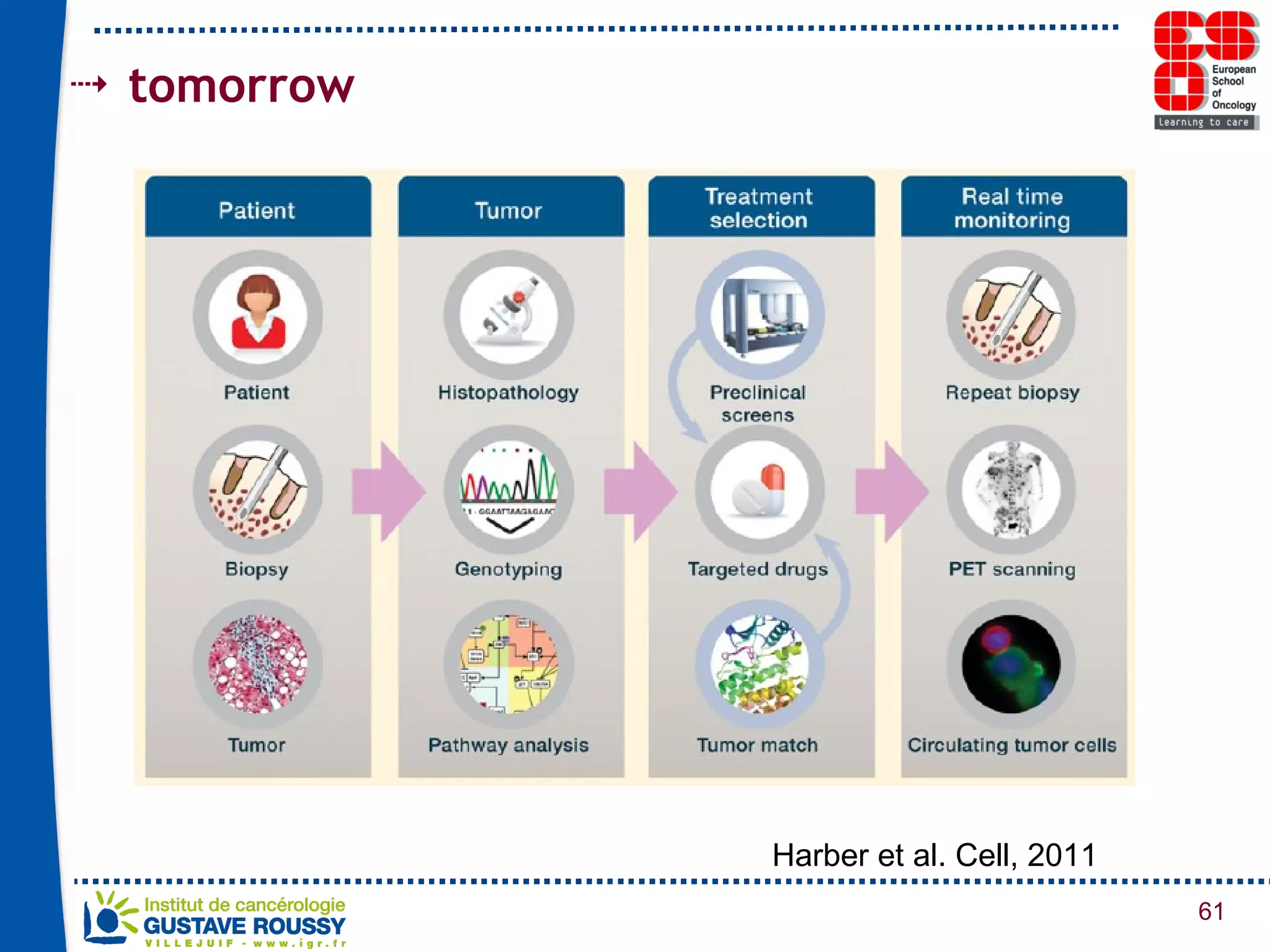
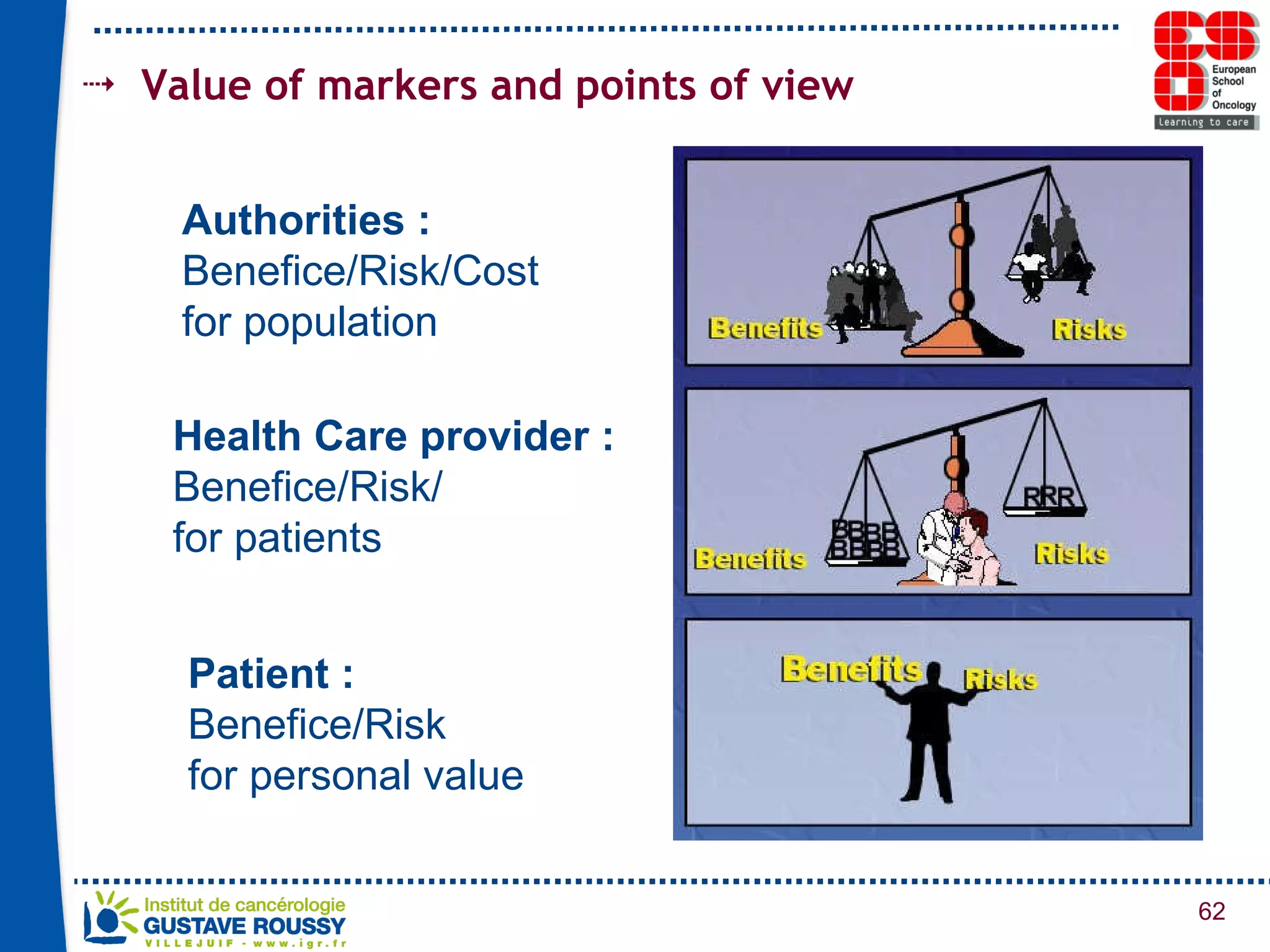
The document summarizes a presentation on using gene profiling and biomarkers to better classify and treat non-small cell lung cancer (NSCLC). It discusses current and emerging markers like EGFR mutations, ALK translocations, and FGFR1 amplifications that define NSCLC subtypes and can guide targeted therapies. Integrating multiple genomic analyses may help characterize unknown abnormalities in a third of NSCLC tumors and identify new treatment opportunities.