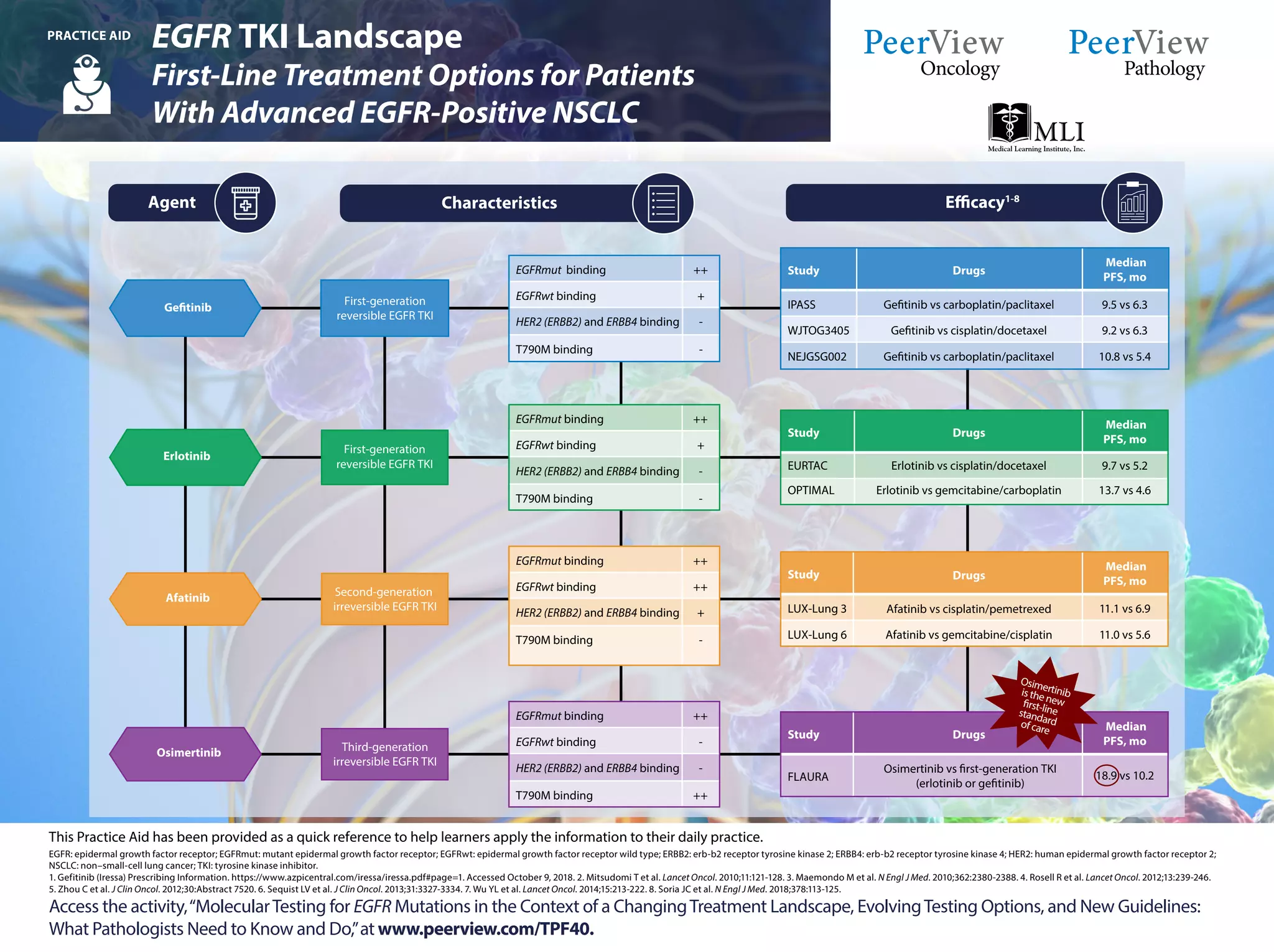
The document outlines guidelines for molecular testing in non-small-cell lung cancer (NSCLC), emphasizing the importance of testing for specific biomarkers like EGFR, ALK, and ROS1 at diagnosis or progression. It discusses the necessity of using clinically validated testing methods, advocating for multiplexed genetic sequencing over single-gene tests, and addresses which mutations should be prioritized in patients. Furthermore, it highlights the evolving treatment landscape and provides recommendations related to various targeted therapies and testing methodologies.