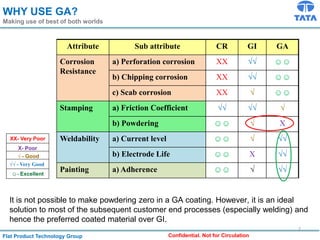
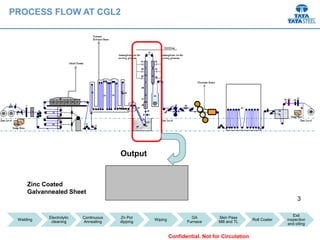
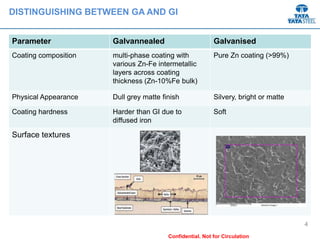
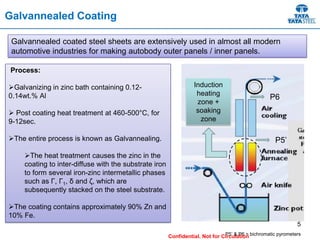
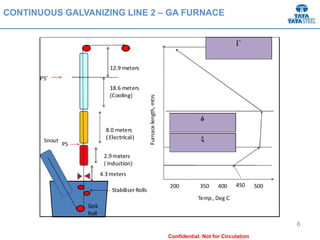
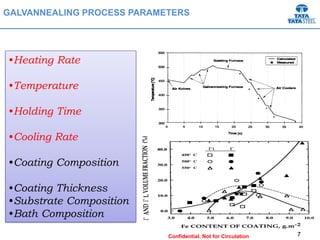
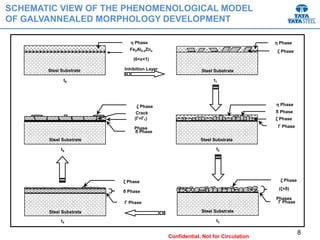
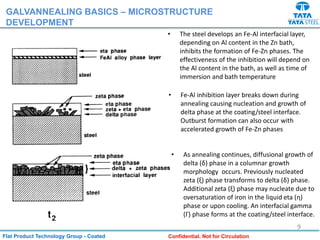
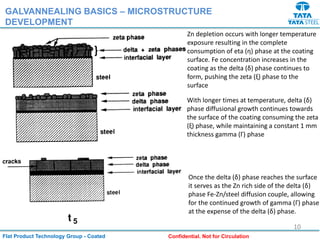
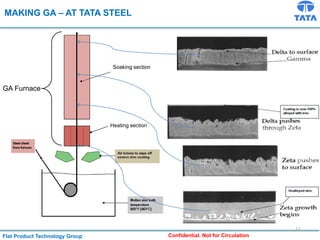
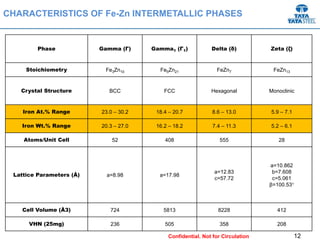
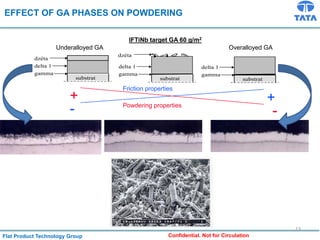
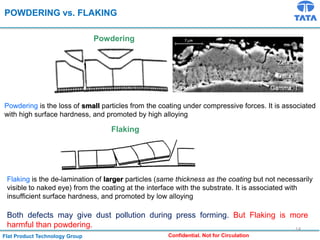
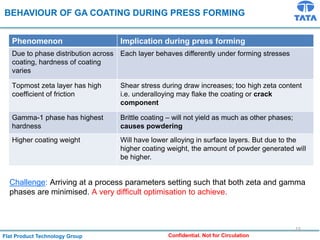
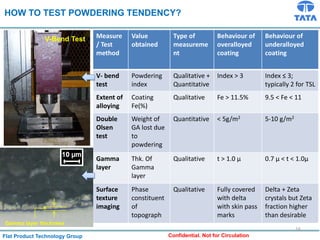
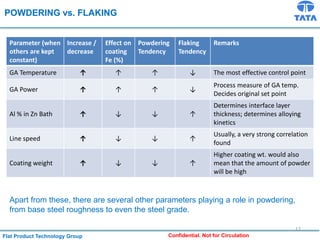
Galvannealed coating provides corrosion resistance, good stamping and welding properties, and good paint adherence. It has a dull grey appearance due to its multi-phase zinc-iron coating. While it cannot eliminate powdering completely, it is preferred over galvanized coating for most subsequent processes. The galvannealing process involves zinc coating followed by heat treatment to form zinc-iron intermetallic phases. Key process parameters include temperature, time, coating thickness and composition, which determine the phase development and resulting properties like corrosion resistance and powdering tendency.