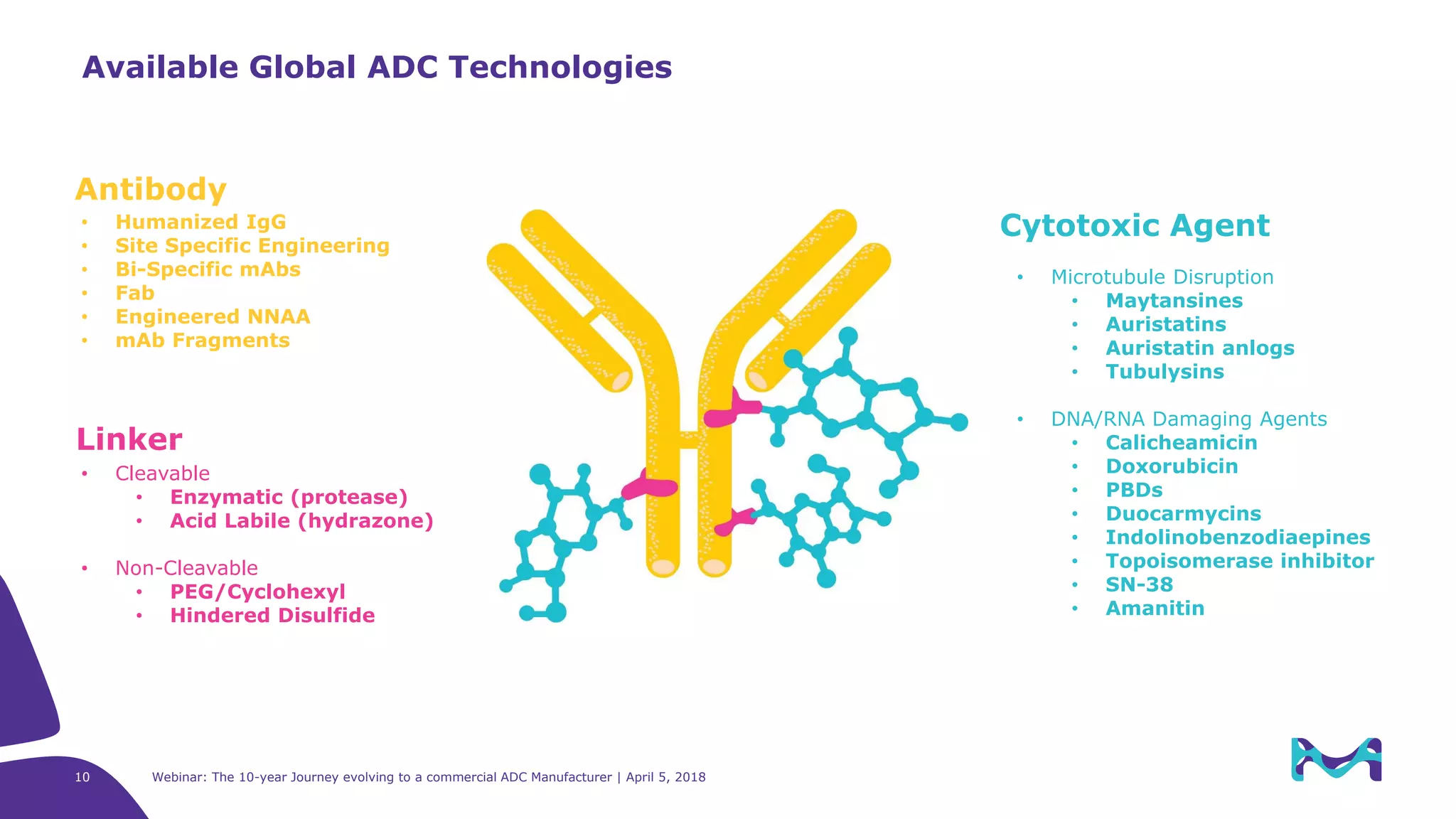
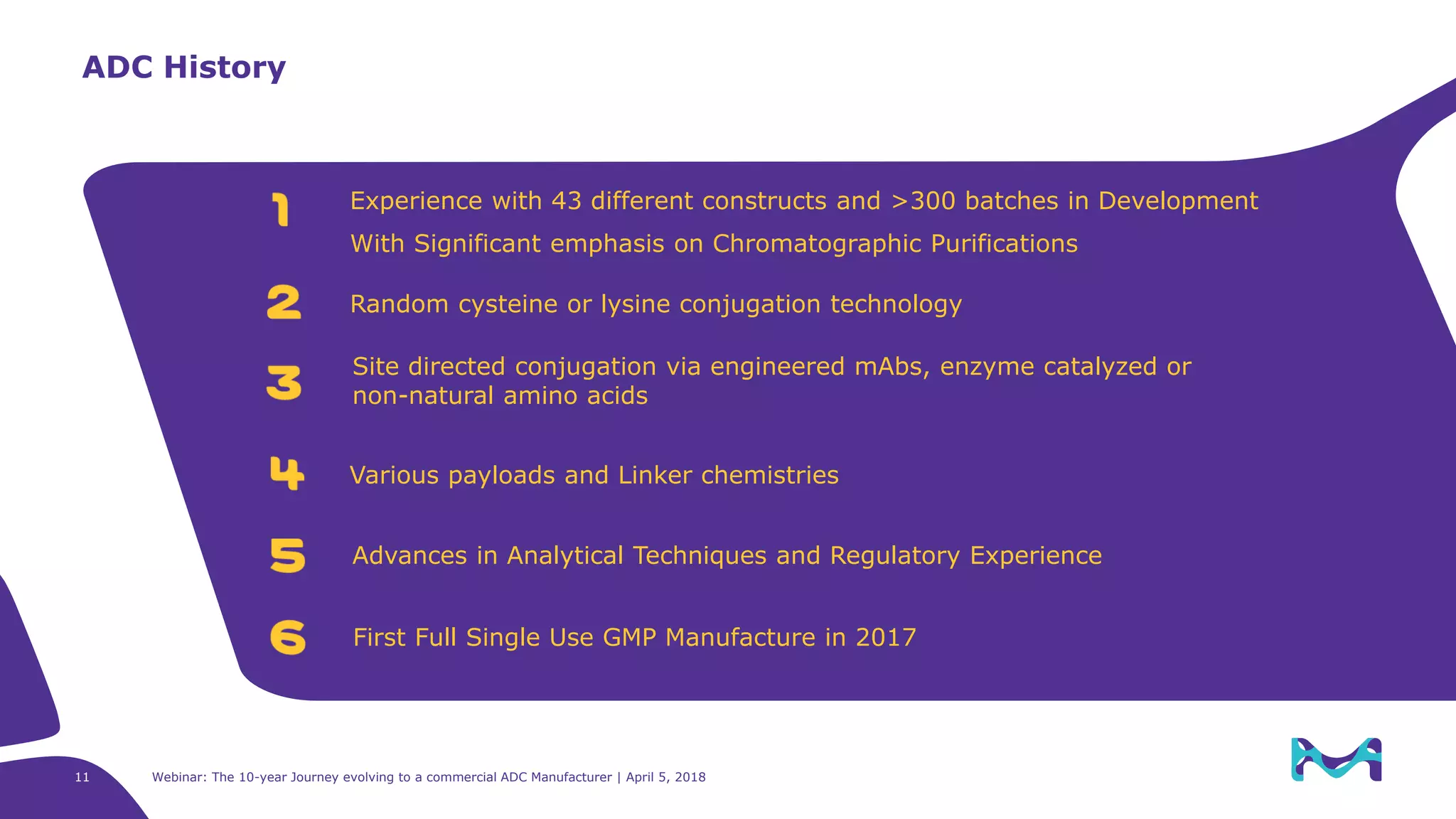
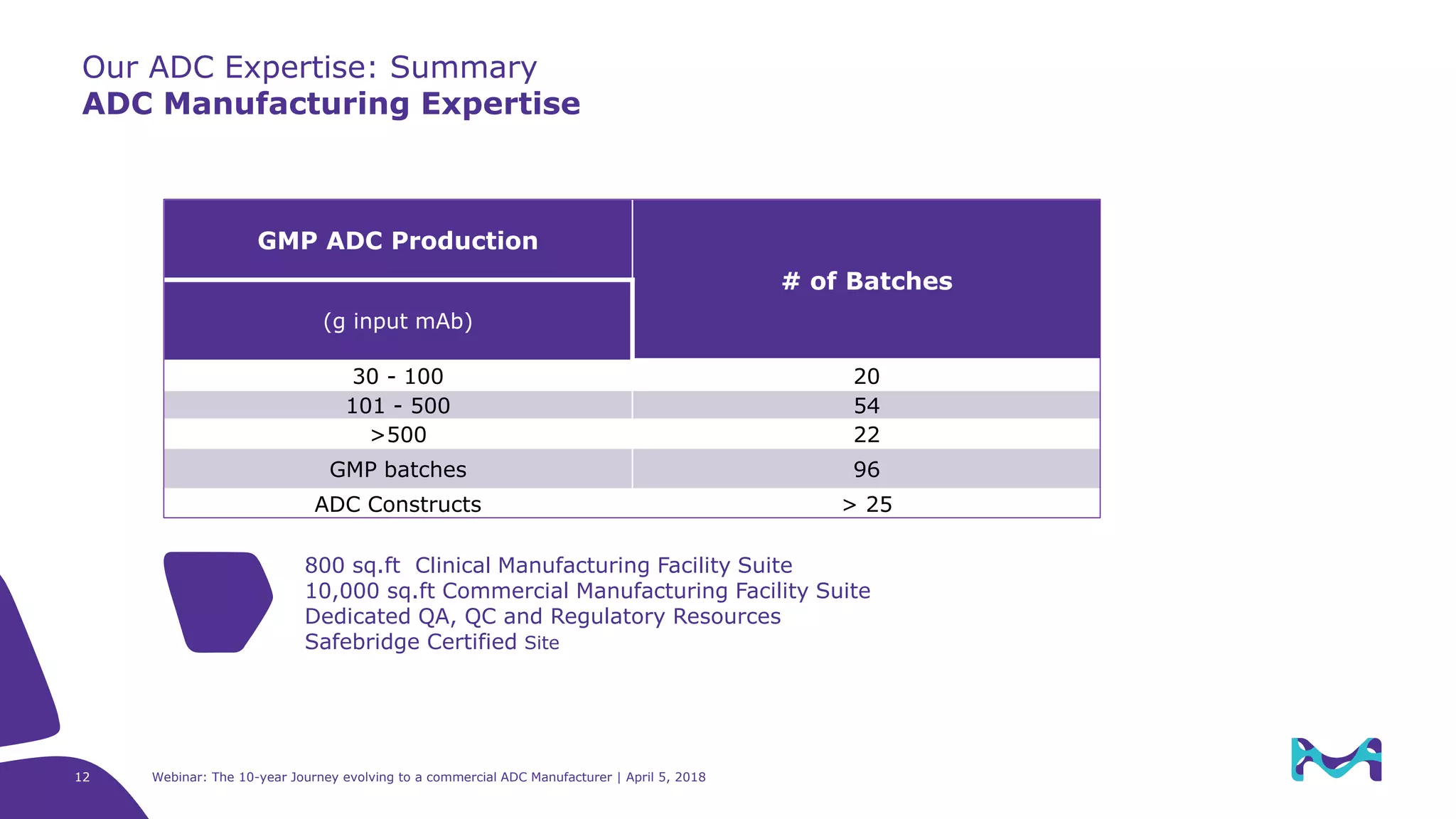
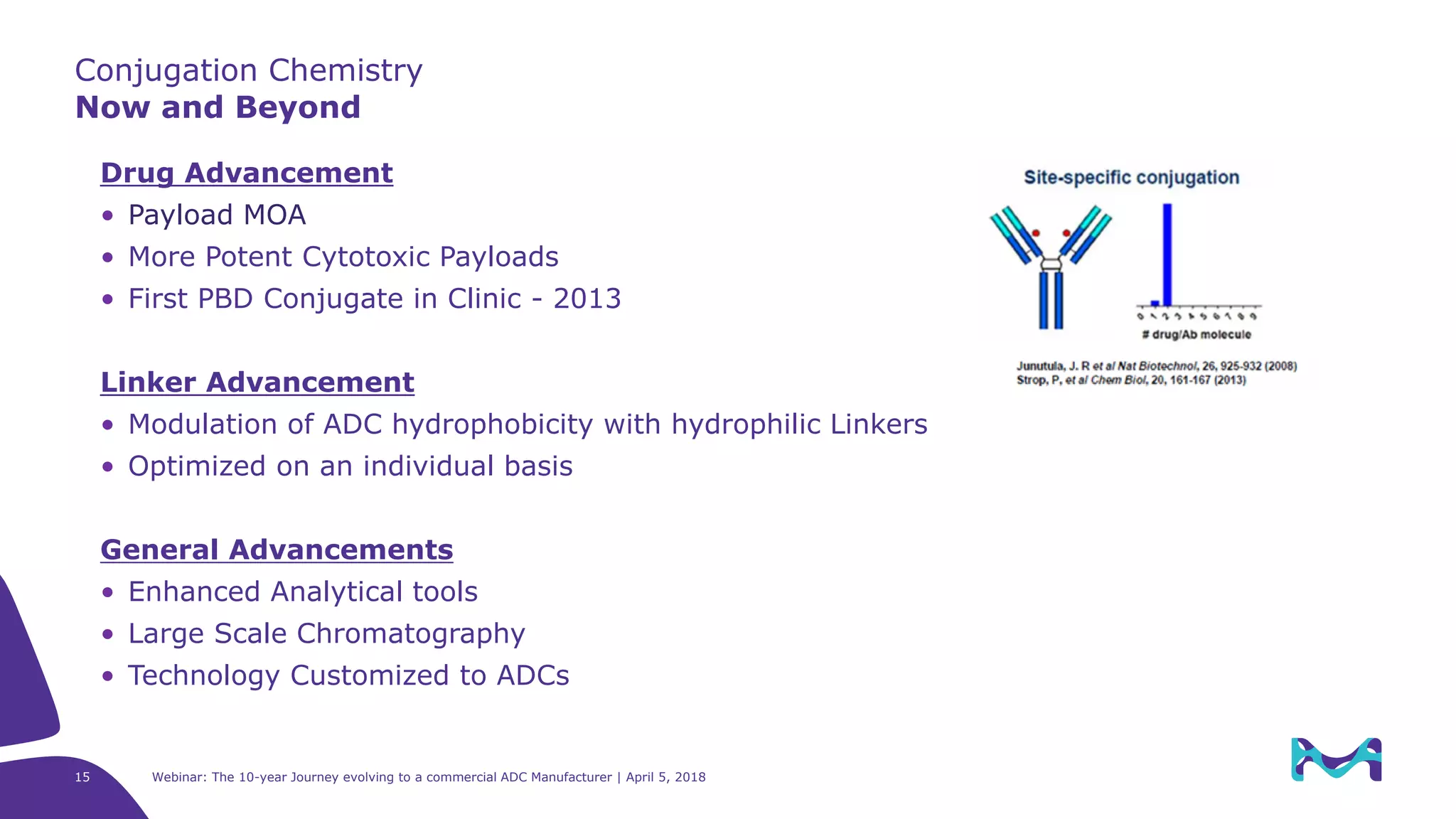
The document details the evolution of antibody-drug conjugates (ADCs) and Merck KGaA's journey to becoming a commercial ADC manufacturer over the past decade. It discusses the advancements in ADC technology, including the types of antibodies, linkers, and payloads used, and the global ADC pipeline with numerous clinical and pre-clinical programs. The webinar also highlights the challenges in manufacturing ADCs and the future directions for technology and regulatory considerations.