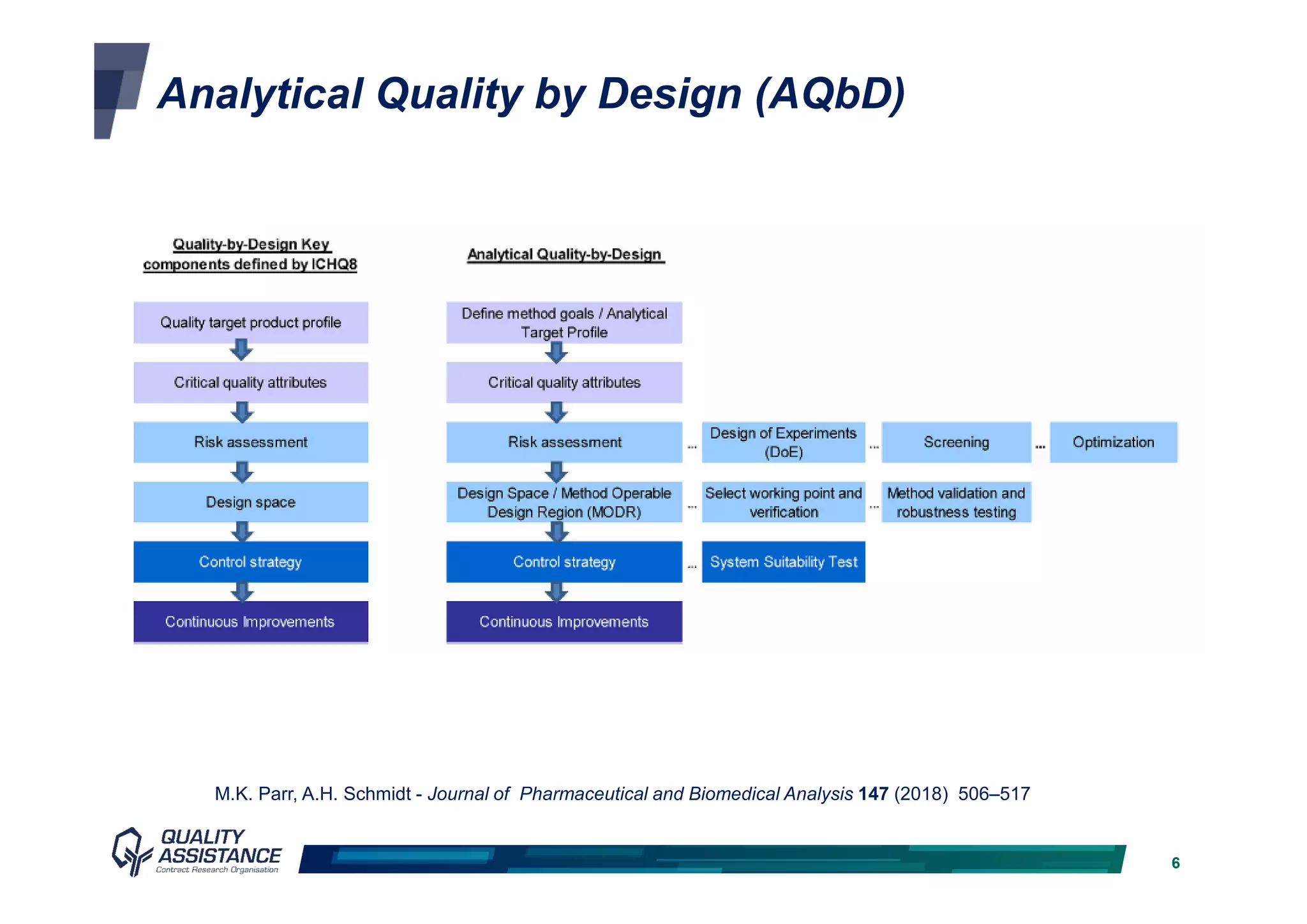
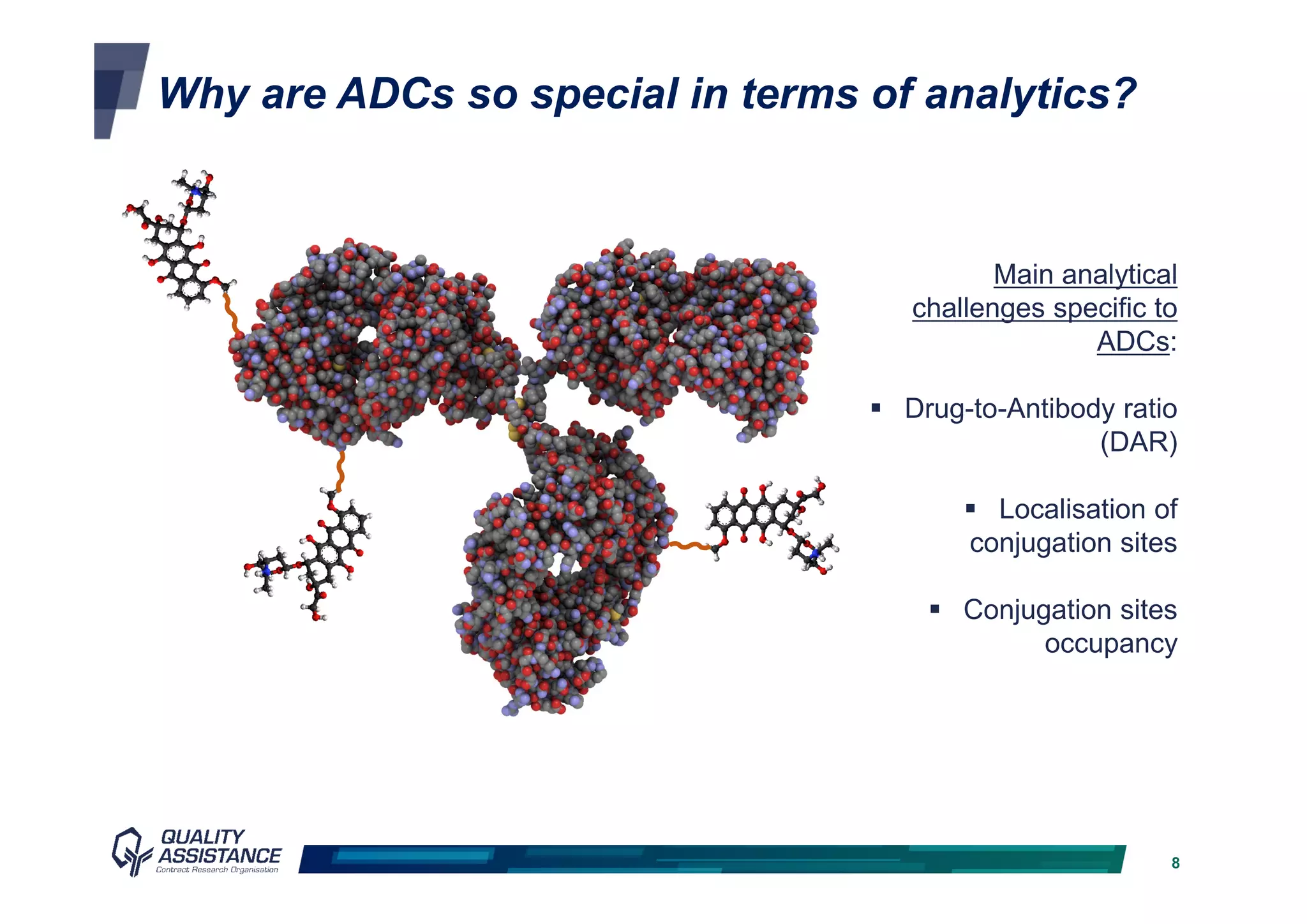
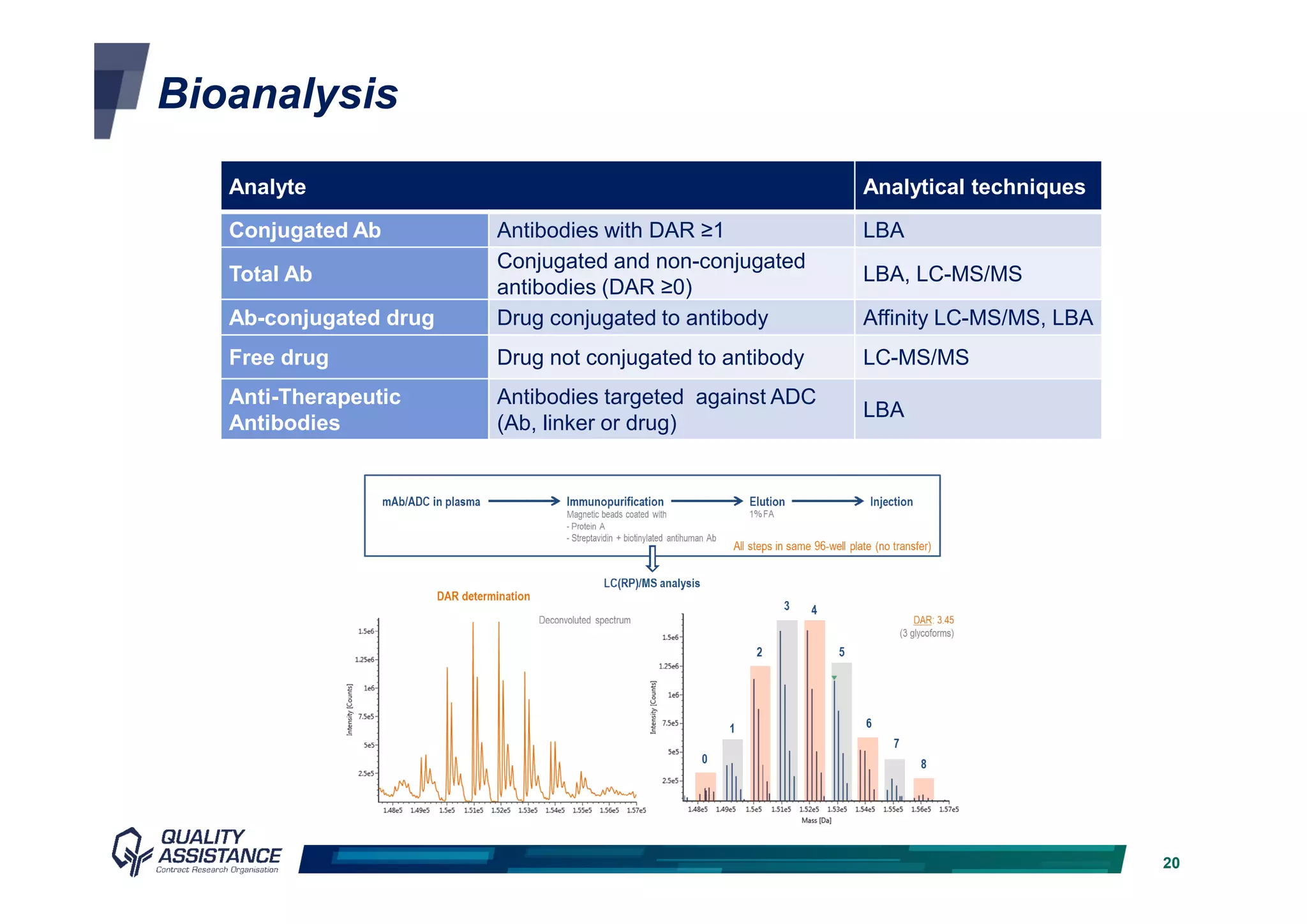
The document discusses analytical methods and challenges in bringing antibody-drug conjugates (ADCs) to market, emphasizing the importance of specific evaluation metrics like drug-to-antibody ratio and conjugation site characterization. It outlines the analytical method lifecycle from preclinical stages through post-licensure, highlighting the need for compliance with EMA/FDA regulations. The text also underscores the importance of developing sensitive analytical techniques for ensuring product safety and efficacy within ADC design and analysis.