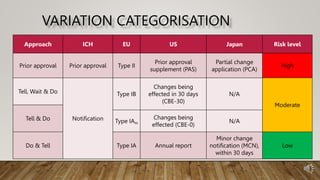
The document outlines the categorization of post-approval CMC changes in product lifecycle management (PLCM) according to ICH Q12, focusing on regulatory mechanisms, systems, and risk-based categorization. It distinguishes between high-risk changes requiring prior approval and moderate to low-risk changes that only require notification to authorities. Additionally, the document emphasizes the importance of harmonizing categorization processes across different regulatory jurisdictions.