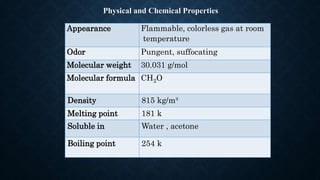
Formaldehyde is the simplest aldehyde with the formula CH2O. It is a reactive, colorless gas that was first synthesized in 1859 and is now a major industrial chemical. Formaldehyde is produced industrially by the catalytic oxidation of methanol in a process called the formox process. It has many uses including as a preservative, embalming agent, and in the production of resins, plastics, and other chemicals. Exposure to high levels of formaldehyde can cause health issues like irritation of the eyes, nose and throat.






![Uses of Formaldehyde
1] Preservative in medical laboratories.
2] Embalming agent in mortuaries
3] Manufacture of urea , phenol, and melamine resins and for a variety of
special industrial chemicals.
4] Adhesives in the manufacture of particle board , fiberboard, and
plywood , and for molding, paper treating and coating , textile treating ,
surface coating and foams for insulation .
5] Formaldehyde is also used as a treatment for athlete's foot, in cough
drops , skin disinfectants, mouthwashes , spermatic creams, as a
disinfectants for vasectomies and root canals.](https://image.slidesharecdn.com/alkamamppt-210201083746/85/formaldehyde-8-320.jpg)

