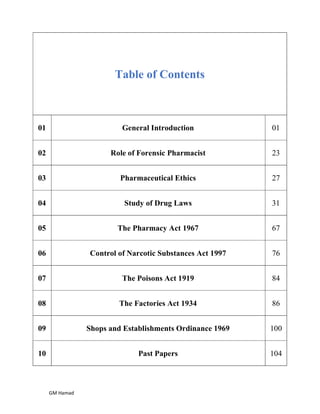
The document provides an overview of forensic pharmacy and pharmacy education in Pakistan. It discusses the role of forensic pharmacists in legal cases and summarizes Pakistan's drug legislation history including key acts like the Drugs Act 1976 and the DRAP Act 2012. It also outlines the objectives and preparation of Pakistan's National Essential Drugs List, and describes the drug control administration at federal and provincial levels as well as the registration process for drugs.