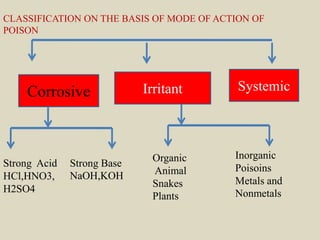
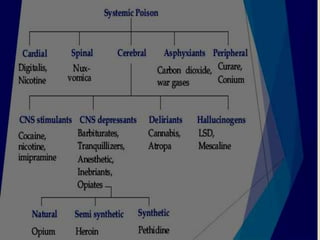
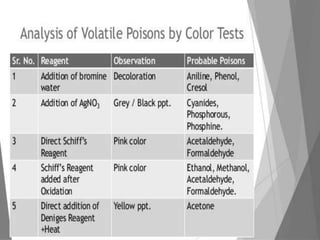
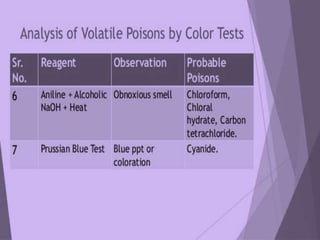
The document discusses methods for analyzing toxic substances and poisons. It describes:
- LD50 testing which measures the lethal dose (LD) of a substance required to kill 50% of test subjects. This allows comparison of relative toxicities.
- Extraction methods used to isolate acidic, basic, volatile, and non-volatile poisons from tissues through use of solvents and acid-base reactions.
- Various analytical techniques like TLC, GC, AAS that can identify specific poisons isolated from samples based on their chemical properties.
- Examples of tests used to detect common poisons like ethanol, barbiturates, DDT, and methods to analyze heavy metals like mercury and arsenic