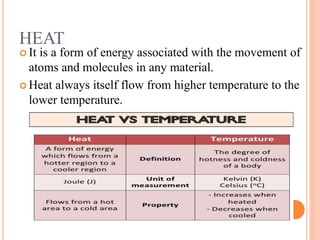
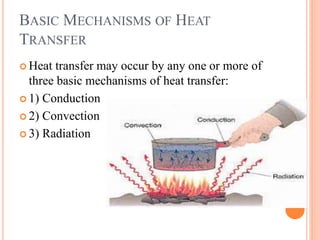
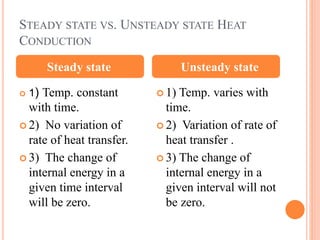
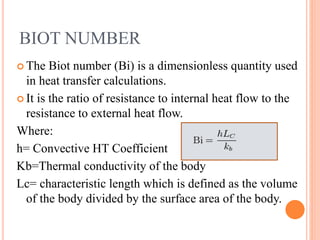
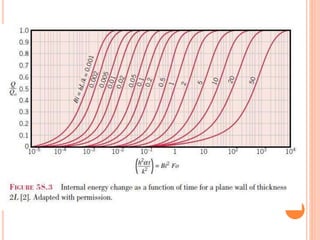
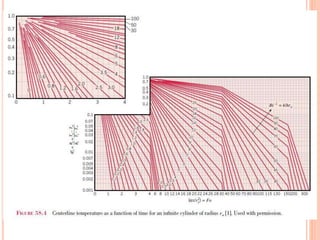
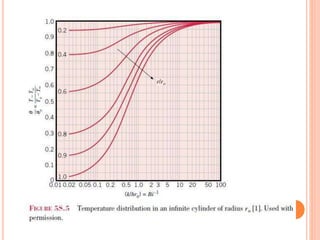
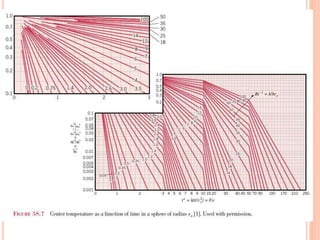
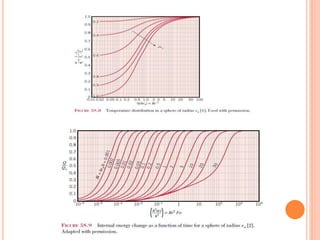
This document summarizes a presentation on heat transfer mechanisms including conduction, convection, and radiation. It discusses key concepts like temperature, heat, the three mechanisms of heat transfer, conduction, Biot number, Fourier number, and Heisler charts. Conduction involves transfer of kinetic energy between adjacent molecules. Biot and Fourier numbers are dimensionless parameters used in heat transfer calculations to characterize conduction regimes and transient behavior. Heisler charts provide a graphical tool to analyze heat transfer problems.